A) H2CO
B) NO2-
C) C2F4
D) H2CCO
E) PO43-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule or ion is not planar?
A) XeF4
B) NO3-
C) BCl3
D) F2CCF2
E) CF4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule or ion does not have a trigonal pyramidal molecular geometry?
A) AsF3
B) NF3
C) PF3
D) BF3
E) IO3-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule or ion has the highest bond order?
A) F2-
B) O2
C) O22-
D) N2
E) F2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The nitrosyl ion,NO+,has ten bonding electrons and four antibonding electrons.Therefore,it has a bond order of
A) 1.
B) 5/2.
C) 7.
D) 2.
E) 3.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The approximate H-C-C bond angle in ethane,C2H6,is
A) 60°.
B) 180°.
C) 120°.
D) 109°.
E) 90°.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In ClF3,the electron pairs are arranged about the chlorine atom in
A) a square plane.
B) a tetrahedron.
C) an octahedron.
D) a trigonal pyramid.
E) a trigonal bipyramid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is incorrect regarding the water molecule?
A) There are two lone pairs and two bonding pairs on the central atom.
B) The molecule is polar.
C) The hybridization of oxygen is sp3.
D) The hybridization of hydrogen is sp.
E) The molecular geometry is bent.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry around carbon atom C1?
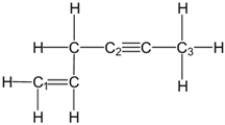
A) tetrahedral
B) trigonal planar
C) linear
D) trigonal pyramidal
E) bent
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following species has(have) a bond order of 2? 1) NO- 2.O2 3.O2-
A) 1 only
B) 3 only
C) 2 only
D) 1 and 3
E) 1 and 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many sigma and pi bonds are in the molecule pictured below? 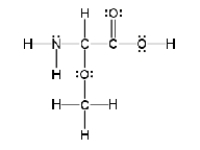
A) thirteen sigma bonds and one pi bond
B) eleven sigma bonds and two pi bonds
C) thirteen sigma bonds and two pi bonds
D) eleven sigma bonds and five pi bonds
E) five sigma bonds and eleven pi bonds
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements best describes N2O?
A) The molecular geometry is bent and the molecule is nonpolar.
B) The molecular geometry is linear and the molecule is nonpolar.
C) The molecular geometry is linear and the molecule is polar.
D) The molecular geometry is trigonal planar and the molecule is nonpolar.
E) The molecular geometry is bent and the molecule is polar.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For which molecule or ion does the nitrogen atom have the positive end of the dipole moment?
A) NH4+
B) CN−
C) NO
D) HCN
E) N2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the bond angle in a trigonal planar molecule or ion?
A) 109°
B) 180°
C) 90°
D) 72°
E) 120°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule or ion has a trigonal planar molecular geometry?
A) PCl3
B) HCN
C) CO32-
D) HCCH
E) AsF3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule or ion does not have a trigonal pyramidal molecular geometry?
A) PO33-
B) SO32-
C) NI3
D) BF3
E) XeO3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the molecular orbital diagram for dinitrogen (N2) excluding the K shells below,which of the following molecules or ions is expected to be diamagnetic? 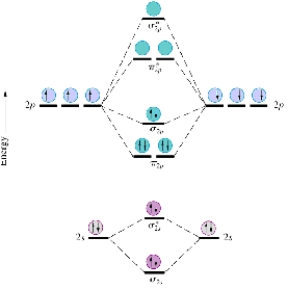
A) C22-
B) O2
C) B2
D) O2-
E) O2+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the electron geometry (or electron arrangement) around an atom in a molecule or ion which is surrounded by three lone pairs of electrons and one single bond.
A) tetrahedral
B) trigonal pyramidal
C) trigonal planar
D) bent
E) linear
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of the nitrogen atom in the nitrite ion?
A) sp3d
B) sp3
C) s
D) sp
E) sp2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molecular geometry of the ammonium ion,NH4+,is most similar to the molecular geometry of
A) NH3.
B) CH4.
C) N2H4.
D) NH2-.
E) CH3+.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 101
Related Exams