A) NO2+
B) OCN-
C) NO2-
D) NO
E) SO3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the C-C-H bond angle in H2CCO?
A) 109°
B) 180°
C) 120°
D) 144°
E) 90°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the ICl4- ion,the electron pairs are arranged around the central iodine atom in the shape of
A) a tetrahedron.
B) an octahedron.
C) a square plane.
D) a trigonal bipyramid.
E) a trigonal pyramid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the molecular orbital diagram for dihydrogen (H2) below,what would be the bond order of He2+
?
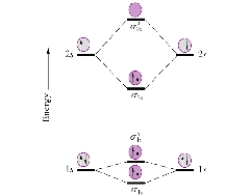
A) ![]()
B) ![]()
C) 1
D) 0
E) 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of Se in SeF6?
A) sp3d
B) sp3d2
C) sp2
D) sp
E) sp3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When an atom in a molecule or ion is described as sp3d1 hybridized,its molecular geometry is
A) trigonal bipyramidal.
B) trigonal planar.
C) linear.
D) tetrahedral.
E) octahedral.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule or ion does not have a tetrahedral molecular geometry?
A) BF4-
B) NF4+
C) GeF4
D) XeF4
E) BeF42-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following characteristics does not apply to PF3?
A) has three σ bonds
B) contains polar bonds
C) polar molecule
D) one lone pair of electrons on phosphorus
E) trigonal planar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule does not have a planar molecular geometry?
A) SO3
B) HCCH
C) N2H4
D) HNNH
E) C2F4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the hydronium ion,H3O+,the electron groups are arranged about the central oxygen atom in a
A) tetrahedron.
B) square plane.
C) pyramid.
D) trigonal plane.
E) bent structure.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is nonpolar?
A) H2S
B) XeF2
C) SO2
D) N2O
E) HCl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The approximate CCO angle in acetone, 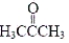 ,is
,is
A) 180°.
B) 90°.
C) 109°.
D) 60°.
E) 120°.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules is nonpolar?
A) SF4
B) PF5
C) ClF3
D) PF3
E) CH2F2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the I3- ion,how many electron groups surround the central atom?
A) 5
B) 3
C) 6
D) 4
E) 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molecule AX3,in which A is the central atom,is polar and obeys the octet rule; therefore,
A) A has two lone pairs.
B) A has one lone pair.
C) A has no lone pairs.
D) A has four bonding pairs.
E) A has three lone pairs.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the labeled carbons (C1-C4) is/are sp-hybridized?
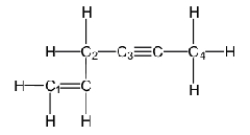
A) carbon three
B) carbon one
C) carbon two and four
D) carbon two
E) carbon one and three
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the electron geometry (or electron arrangement) around an atom in a molecule or ion which is surrounded by three lone pairs of electrons and two single bonds.
A) trigonal bipyramidal
B) see-saw or distorted tetrahedron
C) T-shaped
D) linear
E) trigonal planar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule or ion is not planar?
A) CO32-
B) Cl2CCCl2
C) HNNH
D) H3O+
E) F2CO
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has a dipole moment?
A) SO2
B) CS2
C) ClCCCCCl
D) CCl4
E) HCCH
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry around an atom in a molecule or ion which is surrounded by one lone pair of electrons and four single bonds.
A) see-saw or distorted tetrahedron
B) trigonal bipyramidal
C) linear
D) T-shaped
E) trigonal planar
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 101
Related Exams