A) 180 s
B) 47 s
C) 930 s
D) 18 s
E) 93 s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction A + B + C → products,the following initial-rate data were obtained. [A]0 (mol/L) [B]0 (mol/L) [C]0 (mol/L) Initial Rate (mol/(L ∙ s) ) 0) 40 0) 40 0) 20 0) 0160 0) 20 0) 40 0) 40 0) 0080 0) 60 0) 10 0) 20 0) 0015 0) 20 0) 10 0) 20 0) 0005 0) 20 0) 20 0) 40 0) 0020 What are the reaction orders with respect to A,B,and C,respectively?
A) 0,1,1
B) 1,2,1
C) 1,1,1
D) 1,2,0
E) 0,2,1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction 2H2(g) + 2NO(g) → 2H2O(g) + N2(g) Is first-order in H2 and second-order in NO at a particular temperature.What is the rate law?
A) Rate = k[H2]2[NO]2
B) Rate = k[H2][NO]2
C) Rate = k[H2][NO]
D) Rate = k[H2O]2[N2]
E) Rate = k[H2]2[NO]
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At 500oC,cyclopropane (C3H6) reacts to form its isomer,propene (C3H6) .The reaction is first-order,and the rate constant is 6.7 × 10-4 s-1.If the initial concentration of cyclopropane is 0.500 M and the initial concentration of propene is 0,determine the time required for the concentration of propene to reach 0.100 M.
A) 3.4 × 103 s
B) 3.3 × 102 s
C) 1.2 × 104 s
D) 7.5 × 102 s
E) 2.4 × 103 s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction between selenous acid and the iodide ion in acid solution is H2SeO3(aq) + 6I-(aq) + 4H+(aq) → Se(s) + 2I3-(aq) + 3H2O(l) The data in the following table were measured at 0°C. Experiment [H2SeO3]0 (M) [H+]0 (M) [I-]0 (M) Initial Rate [mol/(L ∙ s) ] 1 1) 00 × 10-4 2) 00 × 10-2 3) 00 × 10-2 5) 30 × 10-7 2 2) 00 × 10-4 2) 00 × 10-2 3) 00 × 10-2 1) 06 × 10-6 3 3) 00 × 10-4 4) 00 × 10-2 3) 00 × 10-2 6) 36 × 10-6 4 3) 00 × 10-4 8) 00 × 10-2 3) 00 × 10-2 2) 54 × 10-5 5 3) 00 × 10-4 8) 00 × 10-2 6) 00 × 10-2 2) 04 × 10-4 6 2) 00 × 10-4 2) 00 × 10-2 6) 00 × 10-2 8) 48 × 10-6 The overall order of this reaction is
A) 4.
B) 6.
C) 2.
D) 8.
E) 3.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a reaction is zero-order in a reactant,when the concentration of the reactant is decreased by a factor of 2,the reaction rate will
A) quadruple.
B) decrease by a factor of 1/2.
C) remain constant.
D) decrease by a factor of 1/4.
E) double.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A suggested mechanism for the decomposition of ozone is
O3 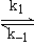 O2 + O fast equilibrium
O + O3
O2 + O fast equilibrium
O + O3 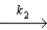 2O2 slow step
When the concentration of ozone is doubled and the concentration of oxygen is doubled,the instantaneous rate
2O2 slow step
When the concentration of ozone is doubled and the concentration of oxygen is doubled,the instantaneous rate
A) increases by a factor of 8.
B) remains the same.
C) increases by a factor of 4.
D) decreases.
E) increases by a factor of 2.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A chemical reaction that is first-order in X is observed to have a rate constant of 2.00 × 10-2 s-1.If the initial concentration of X is 1.0 M,what is the concentration of X after 196 s?
A) 0.20 M
B) 0.020 M
C) 50 M
D) 0.61 M
E) 0.98 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is incorrect?
A) The slow step in a mechanism generally involves a three-body collision.
B) After the addition of a catalyst,the collision rate between molecules is still the same.
C) Most collisions between reactant molecules do not lead to a product.
D) Chemical reactions involve collisions between the participating molecules.
E) A three-body collision is less likely than a two-body collision.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the first-order reaction 1/2 N2O4(g) → NO2(g) ; ΔH = 28.6 kJ The activation energy is 53.7 kJ/mol.What is the activation energy for the reverse reaction?
A) 15.2 kJ/mol
B) 82.3 kJ/mol
C) -53.7 kJ/mol
D) 25.1 kJ/mol
E) 53.7 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The catalyzed pathway in a reaction mechanism has a __________ activation energy and thus causes a __________ reaction rate.
A) higher,lower
B) higher,higher
C) higher,steady
D) lower,higher
E) lower,steady
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nitrosyl chloride is produced from the reaction of nitrogen monoxide and chlorine: 2NO(g) + Cl2(g) → 2NOCl(g) The following initial rates at a given temperature were obtained for the concentrations listed below. Experiment Initial Rate (mol·L-1·h-1) [NO]0 (mol·L-1) [Cl2]0 (mol·L-1) 1 2) 21 0) 25 0) 25 2 19) 89 0) 75 0) 25 3 6) 63 0) 25 0) 75 From the data,what is the experimental rate law?
A) Rate = k[Cl2]
B) Rate = k[NO]
C) Rate = k[NO][Cl2]2
D) Rate = k[NO]2[Cl2]
E) Rate = k[NO][Cl2]1/2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the elementary reaction A + B → C + D,what is the predicted rate law?
A) Rate = k[A]2
B) Rate = k[A][B]
C) Rate = k ![]()
D) Rate = k[B]2
E) Rate = k[A]/[C]
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a certain reaction of the general form aA → products,a plot of the experimental data as [A] versus time is linear.What is the reaction order with respect to reactant A?
A) zero
B) first
C) second
D) fourth
E) third
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is incorrect?
A) The rates of most chemical reactions change with time.
B) The rate constant for a reaction can be changed by changing the temperature.
C) The rate constant is dependent on the reactant concentrations.
D) In a series of stepwise reactions,the rate-determining step is the slowest one.
E) The rate of a catalyzed reaction is dependent on the concentration of the catalyst.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The hypochlorite ion oxidizes the iodide ion in aqueous solution as represented by the following equation:
OCl-(aq) + I-(aq) → OI-(aq) + Cl-(aq)
The rate law for this reaction is Rate = k 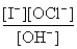 ) The overall reaction order and the order with respect to OH- are
) The overall reaction order and the order with respect to OH- are
A) 2 and -1.
B) 0 and -1.
C) 0 and 1.
D) 2 and 1.
E) 1 and -1.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the hypothetical first-order reaction A → products,k = 0.0822 s-1.If the initial concentration of A is 0.372 M,how long would it take for A to be 28.2% consumed?
A) 8.43 s
B) 4.03 s
C) 12 s
D) 32.7 s
E) 15.3 s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The nuclide 188W decays by a first-order process with a rate constant of 1.0 × 10-2 d-1.How long will it take for 88% of the initial amount of 188W to be consumed?
A) 6 d
B) 240 d
C) 12 d
D) 210 d
E) 92 d
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the hypothetical reaction A + 2B → 2C + D,the initial rate of disappearance of A is 2.0 × 10-2 mol/(L ∙ s) .What is the initial rate of disappearance of B?
A) 8.0 × 10-2 mol/(L ∙ s)
B) 4 × 10-2 mol/(L ∙ s)
C) 1.4 × 10-1 mol/(L ∙ s)
D) 4.0 × 10-4 mol/(L ∙ s)
E) 1.4 × 10-2 mol/(L ∙ s)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction between selenous acid and the iodide ion in acid solution is H2SeO3(aq) + 6I-(aq) + 4H+(aq) → Se(s) + 2I3-(aq) + 3H2O(l) The data in the following table were measured at 0°C. Experiment [H2SeO3]0 (M) [H+]0 (M) [I-]0 (M) Initial Rate [mol/(L ∙ s) ] 1 1) 00 × 10-4 2) 00 × 10-2 3) 00 × 10-2 5) 30 × 10-7 2 2) 00 × 10-4 2) 00 × 10-2 3) 00 × 10-2 1) 06 × 10-6 3 3) 00 × 10-4 4) 00 × 10-2 3) 00 × 10-2 6) 36 × 10-6 4 3) 00 × 10-4 8) 00 × 10-2 3) 00 × 10-2 2) 54 × 10-5 5 3) 00 × 10-4 8) 00 × 10-2 6) 00 × 10-2 2) 04 × 10-4 6 2) 00 × 10-4 2) 00 × 10-2 6) 00 × 10-2 8) 48 × 10-6 Tripling the initial concentration of H2SeO3 while holding the initial concentrations of H+ and I- constant increases the rate of the reaction by a factor of
A) 8.
B) 4.
C) 3.
D) 2.
E) 1.
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 113
Related Exams