A) sp3-sp2
B) sp3-sp3
C) sp2-sp2
D) sp-sp2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the correct molecular orbital diagram for carbon monoxide?
A) (σ 2s ↑ ↓ σ 2s* ↑ ↓ π2p ↑ ↓,↑ ↓ σ2p ↑ ↓)
B) (σ 2s ↑ ↓ σ 2s* ↑ ↓ π2p ↑ ↓,↑ ↓ σ2p ↑ π2p* ↑)
C) (σ 2s ↑ ↓ σ 2s* ↑ ↓ π2p ↑ ↓,σ2p ↑ ↓ π2p* ↑ ↓)
D) (σ 2s ↑ ↓ σ 2s* ↑ ↓ π2p ↑,↑,σ2p ↑ ↓ π2p* ↑ ↓)
E) (σ 2s ↑ ↓ σ 2s* ↑ ↓ π2pp ↑ ↓,↑ ↓ π2p* ↑ ↓)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement is correct for the structure shown? 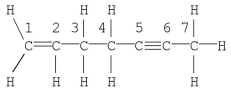
A) Carbon no.1 is described by sp3 hybridization.
B) The molecule contains 19 σ bonds.
C) Carbon no.2 is described by sp2 hybridization.
D) The molecule contains a total of five π bonds.
E) Carbon no.7 is described by sp hybridization.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements concerning molecular orbital (MO) theory is FALSE?
A) The number of MOs formed equals the number of atomic orbitals combined.
B) Any set of MOs from 2 atomic orbitals involves 1 bonding and 1 antibonding orbital.
C) Electrons normally enter the lowest energy MO available to them.
D) No more than two electrons can enter a particular MO.
E) Electrons enter MOs of identical energies in pairs before any enter singly.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following involves delocalized π bonds?
A) acetylene (C2H2)
B) benzene (C6H6)
C) carbon tetrachloride (CCl4)
D) dichlorodifluoromethane (CF2Cl2)
E) ethylene (C2H4)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The hybridization of N in NO2- is ________.
A) sp
B) sp2
C) sp3d
D) sp3
E) sp3d2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The hybridization on Xe in XeF4 is ________.
A) not hybridized
B) sp3
C) sp3d
D) sp3d2
E) sp2d
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of the Xe atom in XeF2?
A) sp3d
B) sp
C) sp3
D) sp2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
π bonds:
A) are the only kind of bonds present in double bonds
B) have very little electron density along the internuclear axis
C) are formed by endwise overlap of p orbits
D) are formed from hybrid orbitals
E) are formed from s orbitals
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of TeCl4?
A) seesaw
B) square planar
C) square pyramidal
D) tetrahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the INCORRECT statement about NH2-
A) There are no π bonds.
B) There are two σ bonds.
C) N is sp3 hybridization.
D) The molecule is bent.
E) There is one lone pair on N.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The HCOO- ion can be described by using delocalized electrons.What is the hybridization of the C atom?
A) sp
B) sp3
C) sp2d
D) sp3d
E) sp2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the correct molecular orbital diagram for O2- ion?
A) (σ 2s ↑ ↓ σ 2s* ↑ ↓ σ2p ↑ ↓ π2p ↑ ↓ π2p* ↑ ↓,↑)
B) (σ 2s ↑ ↓ σ 2s* ↑ ↓ σ2p ↑ ↓ π2p ↑ ↓,↑ ↓ π2p* ↑ ↑,↑)
C) (σ 2s ↑ ↓ σ 2s* ↑ ↓ σ2p ↑ π2p ↑ ↓,↑ ↓ π2p* ↑ ↓)
D) (σ 2s ↑ ↓ σ 2ss* ↑ ↓ σ2p ↑ ↓ π2p ↑ ↓,↑ ↓ π2p* ↑) )
E) (σ 2s ↑ ↓ σ 2ss* ↑ σ2p ↑ ↓ π2p ↑ ↓,↑ ↓,↑ ↓ π2p* ↑)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to MO theory,which is an INCORRECT statement for  ?
?
A) The B.O.is 1/2.
B) There are no unpaired electrons.
C) The σ1s* orbital has one electron.
D) The molecule is paramagnetic.
E) There are 3 electrons in the molecular orbitals.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements concerning the relative energy levels of molecular orbitals for the O2 molecule is INCORRECT?
A) σ1s < σ1s*
B) σ2s < σ2p
C) π2p < σ2p
D) π2p* < σ2p*
E) σ1s* < σ2s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The VSEPR model predicts the H-O-H bond angle in H3O+ to be:
A) 60°
B) 90°
C) less than 109.5° but greater than 90°
D) 109.5°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the INCORRECT statement about HCN.
A) There are two π bonds.
B) There are two σ bonds.
C) N has a lone pair.
D) The molecule is bent.
E) C has sp hybridization.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Three of the molecular shapes which a sp3d hybridized molecule can have are:
A) triangular,trigonal bipyramid,linear
B) linear,square planar,T-shaped
C) irregular tetrahedron,T-shaped,bent
D) T-shaped,linear,trigonal bipyramid
E) linear,tetragonal pyramid,octahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many π-electrons are there in S2O where S is the central atom?
A) 4
B) 2
C) 6
D) 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to molecular orbital (MO) theory,which statement below is true?
A) A molecule with an even number of electrons must be diamagnetic.
B) There are as many sigma bonds as pi bonds in a molecule.
C) There are as many molecular orbitals as there are atomic orbitals.
D) There are as many bonding as antibonding electrons in a molecule.
E) All molecules contain pi bonds.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 104
Related Exams