Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an unsymmetrical reagent?
A) Cl2
B) H2O
C) H2O and HCl
D) HCl
E) I2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major product in the following transformation? 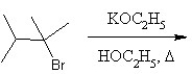
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following steps are a part of chain reaction during alkane substitution? I.propagation II.initiation III.competition IV.dehalogenation V.termination
A) I,II and V
B) I,III and V
C) II,III and V
D) I,IIand IV
E) III,IV and V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Suggest possible compounds for A,B and C in the following scheme: 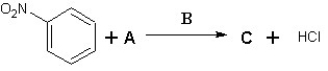
A) A is Cl2,B is AlCl3 and C is meta chloronitrobenzene
B) A is Cl2,B is FeCl3 and C is ortho chloronitrobenzene
C) A is Cl2,B is FeBr3 and C is meta chloronitrobenzene
D) A is HCl,B is AlCl3 and C is para chloronitrobenzene
E) A is Cl2,B is AlBr3 and C is meta chloronitrobenzene
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements correctly describe SN2 and SN1 reactions? I.SN2 reactions proceed with retention of configuration. II.SN1 reactions prefer polar protic solvents. III.SN1 reactions can produce racemic products. IV.SN2 reactions are promoted in the presence of a substrate that produces a very stable carbocation. V.SN1 reactions have a unimolecular rate-determining step.
A) I,IIand V.
B) II,III,and IV.
C) III,IV and V.
D) II,IIIand V.
E) I,III,and IV.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Markovnikof's rule predicts that when HBr is added to an unsymmetrical alkene or alkyne,the H atom will add to which carbon atom?
A) the carbon with the fewest attached hydrogens
B) the carbon with the Br
C) the carbon with the most attached hydrogens
D) the carbon next to the double or triple bond
E) HBr does not react with unsymmetrical alkenes or alkynes
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major product in the reaction between 2-bromo-3-methylbutane and KOtBu in ethanol?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What alkene(s) would be produced in the following reaction? 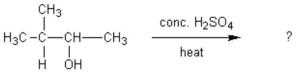
A) 3-methyl-2-pentene
B) 2-methyl-2-butene
C) 3-methylbutene
D) pentene and 1-methylpentene
E) 2-methyl-2-butene and 3-methylbutene
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A hydrocarbon has a molecular weight 56 g/mol and contains 85.7% carbon.It easily adds one mol of HBr to produce a chiral alkyl bromide.Which of the following structures could be the hydrocarbon?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following alcohols can eliminate water to produce alkene? I.phenol II.HOC(CH3) 3 III.HOCH(CH3) 2
A) II and III
B) I ,II and III
C) III only
D) I and II
E) II only
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Lindlar's catalyst is used when ________.
A) we want to reduce alkynes to alkanes
B) we want to speed up HX addition reactions
C) we have to promote elimination reactions over substitution reactions
D) we need to activate Pd hydrogenation catalyst
E) we want to reduce alkynes to Z alkenes
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds could be used as catalysts in halogenation of benzene? I.AlCl3 II.NaCl III.FeBr3 IV.MgCl2 V.PbCl2
A) I and V
B) II and III
C) II and IV
D) I and III
E) III and V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You need to substitute one hydrogen with bromine on an aromatic ring.What reagents do you use?
A) HBr and FeBr3 as catalyst
B) Br2 and heat
C) Br2 and FeBr3 as catalyst
D) a mixture of HBr and conc.H2SO4
E) Br2 and radiation (hυ)
Correct Answer

verified
Correct Answer
verified
True/False
The step reaction polymerizations are fast polymerization reactions.
Correct Answer

verified
False
Correct Answer
verified
True/False
Reactions between alkanes and HX compounds are typical examples of addition reactions.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For which type of reaction is the following an example? 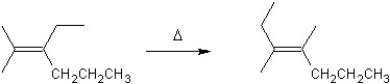
A) substitution
B) elimination
C) polymerization
D) rearrangement
E) addition
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Classify the following polymers as atactic,isotactic or syndiotactic: 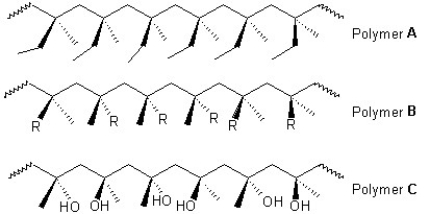
A) Polymer A is isotactic,polymer B is atactic and polymer C is syndiotactic.
B) Polymer A is atactic,polymer B is isotactic and polymer C is syndiotactic.
C) Polymers A and B are isotactic and polymer C is syndiotactic.
D) Polymer A is isotactic and polymers B and C is syndiotactic.
E) Polymer A is syndiotactic,polymer B is isotactic and polymer C is atactic.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Suggest reagents for the synthesis of meta-chloronitrobenzene from nitrobenzene.
A) Cl2 and AlCl3
B) HCl and AlCl3
C) Cl2 and H2SO4
D) HCl and HNO3
E) Cl2 and FeBr3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the product(s) of the reaction (in H2SO4) : CH2=CHCH3 + H2O → product(s)
A) CH2OHCH(OH) CH3
B) CH2OHCH2CH3
C) CH2OHCHOHCH3 + H2
D) CH3CH2CH3 + H2O2
E) CH3CH(OH) CH3
Correct Answer

verified
E
Correct Answer
verified
Showing 1 - 20 of 94
Related Exams