A) release only hydrogen ions.
B) take up only hydrogen ions.
C) release only hydroxide ions.
D) both take up hydrogen ions and release hydroxide ions.
E) release hydrogen and release hydroxide.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An element has an atomic number of 78.The number of protons and electrons in a neutral atom of the element are
A) 156 protons and 78 electrons.
B) 39 protons and 39 electrons.
C) 78 protons and 0 electrons.
D) 78 protons and 78 electrons.
E) 78 protons and 39 electrons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a property of water that results from hydrogen bonding?
A) Ice melts at -100 C.
B) The temperature of water changes very slowly.
C) Many polar substances dissolve in water.
D) Water molecules have cohesiveness.
E) Water has a high surface tension.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT a compound?
A) H2O
B) O?2?
C) NaCl
D) CO2
E) MgCl2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Students were studying properties of water.One student placed a cup containing 80 mL of water in a freezer.Another student placed an identical cup containing 40 mL of water in the same freezer.Which of the following will be the same for both cups of water?
A) the temperature at which the water freezes
B) the mass of the frozen water
C) the time it takes the water to freeze
D) the volume of the frozen water
E) the space it occupies in the cups
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Isotopes of an atom differ in their
A) atomic number.
B) atomic mass.
C) number of electrons.
D) atomic radius.
E) number of protons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would be an example of the value of water's heat capacity?
A) Water is able to travel up a 100 foot tree.
B) Water expands as it freezes causing ice to float on the surface of a lake.
C) Living organisms are better able to maintain their internal body temperature because the temperature of their environment changes very slowly.
D) Small insects can walk on water.
E) Ice cubes float.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom's outermost electron shell
A) is filled when it has three electrons.
B) determines its chemical reactivity.
C) determines its atomic mass.
D) is filled with positively charged particles.
E) is filled identically for every element.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A water molecule,as shown here,is polar because of 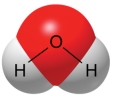
A) transfer of electrons.
B) unequal sharing of electrons.
C) its ability to freeze.
D) its hydrogen bonds.
E) its change in density when frozen.
Correct Answer

verified
Correct Answer
verified
Not Answered
Use the following terms to match the statements provided.

Correct Answer

verified
Correct Answer
verified
Multiple Choice
What do lemons,tomatoes,and coffee all have in common chemically?
A) They are all foods that people consume.
B) They all produce (H+) in solution, making them acids.
C) They all are fruits.
D) They all taste bitter.
E) They are all slippery to the touch.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a way in which chemical bonds can be formed?
A) sharing electrons
B) losing electrons
C) splitting electrons
D) gaining electrons
E) attractions of opposite charge
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best describes the structure of how water molecules form and interact?
A) Hydrogen atoms bond with each other to create stable outer shell of electrons. Then they form a hydrogen bond to an oxygen atom to create the water molecule.
B) Oxygen atoms transfer one electron to each of the hydrogen atoms forming an ionic bond that attracts other water molecules to it.
C) The oxygen atom and hydrogen atoms form a covalent bond with one another to create stable outer shells of electrons. The electrons are shared unequally resulting in a polar molecule whose slight charges form weak hydrogen bond attractions with other water molecules.
D) Hydrogen bonds are formed between the two hydrogen atoms and the oxygen atom. This water molecule than forms a covalent bond with adjacent water molecules.
E) The oxygen atom is more electronegative than the two hydrogen atoms. Due to this, it removes the electron from each hydrogen atom. This satisfies the outer shell of oxygen. Then hydrogen bonds form between the two remaining hydrogen atoms to hold them near to the oxygen atom.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The pH scale is a mathematical indicator of
A) the concentration of H+ present in a solution.
B) the concentration of OH- present in a solution.
C) the total amount of all ions in a solution.
D) the ability of a solution to buffer.
E) the ability to dissolve in water.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom that has an electrical charge is called a(n)
A) ion.
B) molecule.
C) isotope.
D) element.
E) proton.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Baking soda is sometimes used as an antacid.The chemical name for baking soda is Sodium Bicarbonate.What is the bicarbonate doing to help with stomach upset?
A) It is serving as a buffer to take up excess H+ ion from stomach acid.
B) It is able to coat the stomach lining.
C) The bicarbonate helps to create more acid in the stomach.
D) The bicarbonate acts as a strong acid quickly dissociating into H+ ion.
E) It relaxes the stomach muscles.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The pH of pure water is ______ because ___________________________.
A) 7.0; water dissociates an equal number of H+ ions and OH-
B) 14.0; water dissociates and more OH- is formed because there are more hydrogen atoms in water
C) 1.0; water dissociates and more H+ is formed since hydrogen is smaller and can separate from the oxygen easily
D) 7.0; there are no ions formed in pure water
E) acidic; there are more H+ ions than OH- ions present
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How do a strong acid and a weak acid differ?
A) A strong acid has less H+ in solution.
B) A weak acid dissociates only partially in water.
C) A strong acid is less likely to remain dissociated.
D) A weak acid dissociates nearly completely in water.
E) A strong acid dissociates only partly in water.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Some insects can stride on the surface of water because water
A) has a high specific heat.
B) has lower density when frozen.
C) is a good solvent.
D) has surface tension.
E) resists temperature changes.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT a property of water?
A) It is a good solvent.
B) It is denser when frozen than when liquid.
C) It is cohesive.
D) It resists temperature changes.
E) It can be found as a solid, liquid, or gas.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 64
Related Exams