A) metallic crystal.
B) covalent solid.
C) molecular crystal.
D) amorphous solid.
E) ionic crystal.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Potassium crystallizes in a body-centered cubic lattice.How many atoms are there per unit cell?
A) 1
B) 2
C) 4
D) 6
E) 8
Correct Answer

verified
Correct Answer
verified
Short Answer
Indicate all the types of intermolecular forces of attraction in CH2O(g).
Correct Answer

verified
dipole-dip...View Answer
Show Answer
Correct Answer
verified
View Answer
Short Answer
Given that the heat of vaporization of mercury is 59.0 kJ/mol and the vapor pressure of mercury is 0.0017 torr at 25°C, calculate the normal boiling point of mercury.
Correct Answer

verified
380°C (the...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which one of the following substances should exhibit hydrogen bonding in the liquid state?
A) PH3
B) He
C) H2S
D) CH4
E) CH3OH
Correct Answer

verified
Correct Answer
verified
Short Answer
Which of the following liquids would have the lowest viscosity at 25°C? 
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following substances crystallizes as a covalent crystal?
A) CaO
B) SiO2
C) CO2
D) Pb
E) KMnO4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Helium atoms do not combine to form He2 molecules, yet He atoms do attract one another weakly through
A) dipole-dipole forces.
B) ion-dipole forces.
C) dispersion forces.
D) dipole-induced dipole forces.
E) hydrogen bonding.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Platinum has a face-centered cubic crystal structure and a density of 21.5 g/cm3. What is the radius of the platinum atom?
A) 69 pm
B) 98 pm
C) 139 pm
D) 196 pm
E) 277 pm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following data to determine the molar heat of vaporization of chlorine. 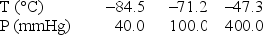
A) 34,700 J
B) 21,900 J
C) 317 J
D) 712 J
E) 9.99 kJ
Correct Answer

verified
Correct Answer
verified
Short Answer
Which is expected to have a higher boiling point, C5H12 or C(CH3)4?
Correct Answer

verified
Correct Answer
verified
Short Answer
Of the given pair of compounds, which would have the higher boiling point? CH3Cl or CH4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The structural form of the element Ge closely resembles the structure of
A) C (diamond) .
B) N (diatomic) .
C) As (tetrahedral) .
D) S (S8 ring) .
E) Kr (monatomic) .
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following crystallizes in a metallic lattice?
A) C
B) NaMnO4
C) K
D) LiClO4
E) K2Cr2O7
Correct Answer

verified
Correct Answer
verified
Short Answer
Copper crystallizes in a face-centered cubic unit cell. The density of copper is 8.94 g/cm3.Calculate the length of the edge of the unit cell in pm.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Each of the following substances is a liquid at -50°C. Place these liquids in order of increasing vapor pressure: dimethyl ether (CH3OCH3) , propane (C3H8) , and ethanol (CH3CH2OH) .
A) ethanol < propane < dimethyl ether
B) ethanol < dimethyl ether < propane
C) propane < dimethyl ether < ethanol
D) dimethyl ether < ethanol < propane
E) propane < ethanol < dimethyl ether
Correct Answer

verified
Correct Answer
verified
Short Answer
Osmium tetroxide, OsO4, is a soft crystal that melts at 40°C.The liquid does not conduct electricity.What kind of crystal is this?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the amount of heat that must be absorbed by 10.0 g of ice at -20°C to convert it to liquid water at 60.0°C. Given: specific heat (ice) = 2.1 J/g·°C; specific heat (water) = 4.18 J/g·°C; Hfus = 6.0 kJ/mol.
A) 420 J
B) 2,900 J
C) 6,300 J
D) 63 kJ
E) 7.5 J
Correct Answer

verified
Correct Answer
verified
Essay
Suppose the atoms in a two-dimensional crystal have the following arrangement: 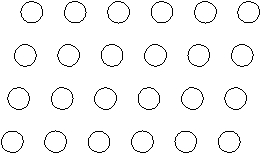 On the drawing above, sketch the unit cell of this crystal.
On the drawing above, sketch the unit cell of this crystal.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How much energy (heat) is required to convert 52.0 g of ice at -10.0°C to steam at 100°C? 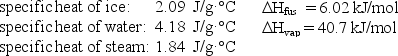
A) 2,570 kJ
B) 1,086 kJ
C) 157.8 kJ
D) 40.2 kJ
E) 22,957 kJ
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 140
Related Exams