A) H2S < H2O < H2Se
B) H2O < H2S < H2Se
C) H2Se < H2O < H2S
D) H2S < H2Se < H2O
E) H2O < H2Se < H2S
Correct Answer

verified
Correct Answer
verified
Short Answer
A solution containing NH3(aq)and NH4Cl(aq)has a pH of 9.5.What is the [NH3]/[NH4+] ratio in this solution? (For ammonia, Kb = 1.8 * 10-5.)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Acid strength decreases in the series HI > HSO4- > HF > HCN. Which of these anions is the weakest base?
A) I-
B) SO42-
C) F-
D) CN-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Acid strength increases in the series: HCN < HF < HSO4-. Which of these species is the strongest base?
A) H2SO4
B) SO42-
C) F-
D) CN -
E) HSO4-
Correct Answer

verified
Correct Answer
verified
Essay
If the pH of pure water is 7.0, what is the hydroxide ion concentration in pure water?
Correct Answer

verified
1 * 10View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Identify the conjugate acid of SO42- in the reaction CO32- + HSO4- 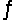 HCO3- + SO42-
HCO3- + SO42-
A) CO32-
B) HSO4-
C) OH-
D) H3O+
E) SO42-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the conjugate base of CH3COOH in the reaction CH3COOH + HSO4- 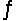 H2SO4 + CH3COO-
H2SO4 + CH3COO-
A) HSO4-
B) SO42-
C) CH3COO-
D) H2SO4
E) OH-
Correct Answer

verified
Correct Answer
verified
Short Answer
Calculate the H+ ion concentration in a solution with a pH of 3.85.
Correct Answer

verified
1.4 * 10View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
The pH of a certain solution is 2.0. How many H+(aq) ions are there in 1.0 L of the solution?
A) 0.01 ions
B) 100 ions
C) 2 ions
D) 6.* 1021 ions
E) 6.* 1023 ions
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The pH of coffee is approximately 5.0. How many times greater is the [H3O+] in coffee than in tap water having a pH of 8.0?
A) 0.62
B) 1.6
C) 30
D) 1,000
E) 1.0 * 104
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The pH of tomato juice is about 4.5. Calculate the concentration of hydrogen ions in this juice.
A) 3.* 10-10 M
B) 3.* 10-5 M
C) 5.* 10-4 M
D) 4.M
E) 3.* 1010 M
Correct Answer

verified
Correct Answer
verified
Short Answer
In comparing three solutions with pH's of 2.0, 4.8, and 5.2, which is most acidic?
Correct Answer

verified
The soluti...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of these species will act as a Lewis acid?
A) NH3
B) NH4+
C) H2O
D) BF3
E) F-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the conjugate base of HCO3- in the reaction CO32- + HSO4- 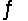 HCO3- + SO42-
HCO3- + SO42-
A) HSO4-
B) CO32-
C) OH-
D) H3O+
E) SO42-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the hydrogen ion concentration in a solution having a pH of 4.60.
A) 4.0 * 10-3 M
B) 4.0 * 10-9 M
C) 4.0 * 10-10 M
D) 2.5 * 10-5 M
E) 2.5 * 10-4 M
Correct Answer

verified
Correct Answer
verified
Essay
HCN is classified as a weak acid in water. What does this classification mean?
Correct Answer

verified
A relatively small f...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Hard water deposits (calcium carbonate) have built up around your bathroom sink. Which one of these substances would be most effective in dissolving the deposits?
A) ammonia
B) bleach (sodium hypochlorite)
C) lye (sodium hydroxide)
D) vinegar (acetic acid)
Correct Answer

verified
Correct Answer
verified
Essay
If the pH of liquid bleach is 12.0, what is the hydroxide ion concentration in this solution?
Correct Answer

verified
1 * 10View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Arrange the acids H2Se, H2Te, and H2S in order of increasing acid strength.
A) H2S < H2Se < H2Te
B) H2S < H2Te < H2Se
C) H2Te < H2S < H2Se
D) H2Se < H2S < H2Te
E) H2Se < H2Te < H2S
Correct Answer

verified
Correct Answer
verified
Short Answer
Will a 0.1 M solution of Na2HPO4(aq)be acidic, basic, or neutral?
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 163
Related Exams