A) a suspension
B) a heterogeneous mixture
C) a solution
D) an element
E) a compound
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How does a suspension differ from a solution?
A) A suspension is a heterogeneous mixture whose components can be separated by simple filtration. A solution is a homogeneous mixture which cannot be separated by simple filtration.
B) A suspension is a heterogeneous mixture consisting of different phases whereas a solution is a homogeneous mixture consisting of a single phase.
C) Although a solution and suspension are both homogeneous mixtures, only the components of a suspension will separate by spinning the mixture in a centrifuge.
D) The difference between a suspension and a solution can only be determined by chemical means.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the winter Vermonters make a tasty treat called "sugar on snow" in which they pour boiled-down maple syrup onto a scoop of clean fresh snow. As the syrup hits the snow it forms a delicious taffy. Which of the following changes are involved in the making of sugar on snow?
A) Boiling down the maple syrup involves the evaporation of water.
B) The syrup warms the snow causing it to melt while the syrup becomes more viscous.
C) As the maple syrup is boiled the sugar within the syrup begins to caramelize, which is an example of a chemical change.
D) All of the above changes are involved in the making of sugar on snow.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following image describes what type of change? 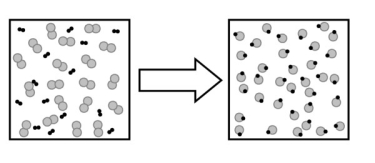
A) a chemical change
B) a physical change
C) a change in state
D) no change
E) an elemental change
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements is a halogen?
A) argon (Ar)
B) lead (Pb)
C) chlorine (Cl)
D) indium (In)
E) lithium (Li)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Helium, He, is a nonmetallic gas and the second element in the periodic table. Rather than being placed adjacent to hydrogen, H, however, helium is placed on the far right of the table because ________.
A) hydrogen and helium repel one another
B) the sizes of their atoms are vastly different
C) they come from different sources
D) helium is most similar to other group 18 elements
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following images could describe an element at the atomic level?
A) ![]()
B) ![]()
C) ![]()
D) none of the images
E) all of the images
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would be considered a homogeneous mixture?
A) wine
B) hydrogen cyanide
C) rusty iron
D) pretzel
E) sugar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Classify the following as element, compound, or mixture, and justify your classifications: sodium chloride, stainless steel, sugar, aluminum, ice.
A) mixture; element; compound; element; element
B) compound; mixture; compound element; compound
C) mixture; compound; mixture; element; compound
D) compound; element; compound; element; compound
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why isn't dirt listed in the periodic table?
A) The periodic table lists only elements made of one kind of material. Dirt is a mixture of elements and compounds.
B) Elements like dirt and air are so common that there is no need to list them in the periodic table.
C) Dirt IS listed in the periodic table but is not easily recognized because it is listed as one of the rare earths with its old scientific name, dysprosium, symbol Dy.
D) None of the above is true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Someone argues that he or she doesn't drink tap water because it contains thousands of molecules of some impurity in each glass. How would you respond in defense of the water's purity, if it indeed does contain thousands of molecules of some impurity per glass?
A) Impurities aren't necessarily bad, in fact, they may be good for you.
B) The water contains water molecules and each water molecule is pure.
C) There's no defense. If the water contains impurities it should not be drunk.
D) Compared to the billions and billions of water molecules, a thousand molecules of something else is practically nothing.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an example of a chemical change?
A) gasoline being used in the engine of a car producing exhaust
B) water freezing into ice crystals
C) aftershave or perfume on your skin generating a smell
D) a piece of metal expanding when heated, but returning to original size when cooled
E) breaking a glass window
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Is the air in your house a homogeneous or heterogeneous mixture?
A) homogeneous because it is mixed very well
B) heterogeneous because of the dust particles it contains
C) homogeneous because it is all at the same temperature
D) heterogeneous because it consists of different types of molecules
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of water that is 99.9999 percent pure contains 0.0001 percent impurities. Consider from Chapter 1 that a glass of water contains on the order of a trillion trillion (1 × 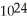 ) molecules. If 0.0001 percent of these molecules were the molecules of some impurity, about how many impurity molecules would this be?
) molecules. If 0.0001 percent of these molecules were the molecules of some impurity, about how many impurity molecules would this be?
A) 1000 (one thousand: 1 × ![]() )
)
B) 1,000,000 (one million: 1 × ![]() )
)
C) 1,000,000,000 (one billion: 1 × ![]() )
)
D) 1,000,000,000,000,000,000 (one million trillion: 1 × ![]() )
)
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Nanoscience has shown that ________.
A) a chemical occurs with the use of a scanning probe microscope
B) the properties of a material can be different on the level of atoms then its properties when the material is analyzed in bulk
C) a physical change can occur when using a scanning probe microscope
D) DNA is the smallest molecule
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements is a transition metal?
A) xenon (Xe)
B) lead (Pb)
C) chlorine (Cl)
D) silver (Ag)
E) lithium (Li)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these properties describes a metal?
A) conducts heat very well
B) brittle
C) fragile
D) transparent
E) doesn't conduct electricity very well
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Compared to microtechnology, nanotechnology focuses on a scale that is about ________.
A) 10 times smaller
B) 100 times smaller
C) 1000 times smaller
D) a million times smaller
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which chemical family is composed almost entirely of synthetic elements?
A) the actinides
B) the lanthanides
C) the halogens
D) all of the above
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a mixture?
A) air
B) gold
C) salt
D) iron
E) helium
Correct Answer

verified
A
Correct Answer
verified
Showing 1 - 20 of 116
Related Exams