A) Br
B) O
C) Ar
D) S
E) Na
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Some products on the market today involving nanotechnology include ________.
A) cars
B) elevators
C) sunscreen
D) cosmetics
E) all of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements is in the fourth period?
A) chromium (Cr)
B) hydrogen (H)
C) beryllium(Be)
D) carbon (C)
E) zirconium (Zr)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following boxes represents a compound? 

 A B C
A B C
A) only A
B) only B
C) only C
D) both A and C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would be considered a heterogeneous mixture?
A) salad dressing
B) water
C) milk
D) vegetable oil
E) vinegar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If you have one molecule of CO2, how many molecules of O2 does it contain?
A) None, O2 is a different molecule than CO2.
B) One, CO2 is a mixture of C and O2.
C) Two, CO2 is a mixture of C and 2 O.
D) Three, CO2 contains three molecules.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of iron weighs more after it rusts because ________.
A) it has expanded into a greater volume
B) rust contains twice as many iron atoms
C) of the additional oxygen it contains
D) Wrong. Iron actually weighs less after it rusts
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following image describes which type of change? 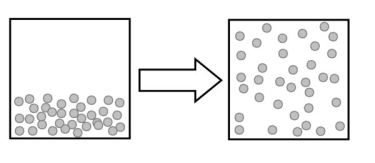
A) a physical change
B) a chemical change
C) an elemental change
D) a change in reactivity
E) no change
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The systematic names for water, ammonia, and methane are dihydrogen monoxide, 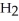 O; trihydrogen nitride, N
O; trihydrogen nitride, N 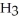 ; and tetrahydrogen carbide, C
; and tetrahydrogen carbide, C 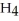 . Why do most people, including chemists, prefer to use the common names for these compounds?
. Why do most people, including chemists, prefer to use the common names for these compounds?
A) The common names are shorter and easier to pronounce.
B) These compounds are encountered frequently.
C) The common names are more widely known.
D) all of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What happens to the properties of elements across any period of the periodic table?
A) The elements tend to become more metallic in nature since they are increasing in atomic number.
B) The elements get much larger in size because of the addition of more protons and electrons.
C) The properties of the elements gradually change across any period of the periodic table.
D) All of the above are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How would you classify the following material? swimming pool water
A) homogeneous mixture
B) heterogeneous mixture
C) a pure element
D) a pure compound
E) depends on how many children have been in it
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following image represents which kind of matter? 
A) a compound
B) a mixture
C) an element
D) none of the above
E) all of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Colorado River water in Colorado has a salinity of about 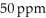 . By the time this water passes into Mexico its salinity has increased to about
. By the time this water passes into Mexico its salinity has increased to about 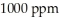 . How many milligrams of salts have been added to each liter of water?
. How many milligrams of salts have been added to each liter of water?
A) 95 milligrams have been added to each liter of water.
B) 950 milligrams have been added to each liter of water.
C) 9500 milligrams have been added to each liter of water.
D) 9.5 milligrams have been added to each liter of water.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would not be considered a chemical property?
A) the temperature at which a liquid will boil
B) light sensitivity
C) whether a metal will rust or not
D) whether a material will dissolve in acid or not
E) the tendency of a material to explode
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How would you classify the following material? coffee (with milk)
A) a suspension
B) a heterogeneous mixture
C) a solution
D) an element
E) a compound
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which atom is smallest?
A) Be
B) Mg
C) Ca
D) Sr
E) All are the same size.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A chemical reaction is synonymous to a ________.
A) physical reaction
B) change in phase
C) rise in temperature
D) chemical change
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these does not describe a metal at room temperature?
A) gas
B) solid
C) liquid
D) shiny
E) bendable
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How might you separate a mixture of sand and salt?
A) with tweezers and a magnifying glass
B) just add water
C) heat the mixture until one of the components melts
D) Two of the above answers are reasonable.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is an example of a physical change?
A) water boiling and being converted into steam
B) water being electrolyzed and being converted in hydrogen and oxygen
C) iron metal reacting with oxygen to form rust
D) a candy bar being digested by a student
E) charcoal being converted into ash
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 116
Related Exams