A) 1
B) 2
C) 3
D) 4
E) 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The peroxydisulfate ion (S2O82-) reacts with the iodide ion in aqueous solution via the reaction:
S2O82- (aq) + 3I- → 2SO4 (aq) + I3- (aq)
An aqueous solution containing 0.050 M of S2O82- ion and 0.072 M of I- is prepared, and the progress of the reaction followed by measuring [I-]. The data obtained is given in the table below. ![The peroxydisulfate ion (S<sub>2</sub>O<sub>8</sub><sup>2-</sup>) reacts with the iodide ion in aqueous solution via the reaction: S<sub>2</sub>O<sub>8</sub><sup>2-</sup> (aq) + 3I<sup>-</sup> → 2SO<sub>4</sub> (aq) + I<sub>3</sub><sup>-</sup> (aq) An aqueous solution containing 0.050 M of S<sub>2</sub>O<sub>8</sub><sup>2-</sup> ion and 0.072 M of I<sup>-</sup> is prepared, and the progress of the reaction followed by measuring [I<sup>-</sup>]. The data obtained is given in the table below. -The average rate of disappearance of I<sup>-</sup> between 1200.0 s and 1600.0 s is ________ M/s. A) 1.8 × 10<sup>-5</sup> B) 1.2 × 10<sup>-5</sup> C) 2.0 × 10<sup>-5</sup> D) 5.0 × 10<sup>4</sup> E) 1.6 × 10<sup>-4</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_4d37_a2ab_fd7b3e9826f6_TB2701_00_TB2701_00_TB2701_00.jpg) -The average rate of disappearance of I- between 1200.0 s and 1600.0 s is ________ M/s.
-The average rate of disappearance of I- between 1200.0 s and 1600.0 s is ________ M/s.
A) 1.8 × 10-5
B) 1.2 × 10-5
C) 2.0 × 10-5
D) 5.0 × 104
E) 1.6 × 10-4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The isomerization of methylisonitrile to acetonitrile CH3NC (g) → CH3CN (g) Is first order in CH3NC. The half-life of the reaction is 2.70 × 104 s at 463 K. The rate constant when the initial [CH3NC] is 0.030 M is ________ s-1.
A) 3.90 × 104
B) 1.23 × 10-3
C) 2.57 × 10-5
D) 8.10 × 102
E) 1.25 × 107
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The elementary reaction 2NO2 (g) → 2NO (g) + O2 (g) Is second order in NO2 and the rate constant at 660 K is 5.23 M-1s-1. The reaction half-life at this temperature when [NO2]0 = 0.45 M is ________ s.
A) 2.4
B) 7.6
C) 0.19
D) 0.13
E) 0.42
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The half-life of a first-order reaction is 13 min. If the initial concentration of reactant is 0.13 M, it takes ________ min for it to decrease to 0.085 M.
A) 12
B) 10.
C) 8.0
D) 11
E) 7.0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The data in the table below were obtained for the reaction:
2 ClO2 (aq) + 2 OH- (aq) → ClO3- (aq) + ClO2- (aq) + H2O (1) 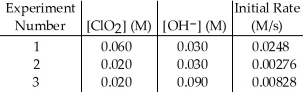 -What is the overall order of the reaction?
-What is the overall order of the reaction?
A) 4
B) 0
C) 1
D) 2
E) 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) →B(g) . The following data are obtained for [A] as the reaction proceeds: ![A flask is charged with 0.124 mol of A and allowed to react to form B according to the reaction A(g) →B(g) . The following data are obtained for [A] as the reaction proceeds: -How many moles of B are present at 10 s? A) 0.011 B) 0.220 C) 0.110 D) 0.014 E) 1.4 × 10<sup>-3</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_2626_a2ab_9b6fd935b612_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00_TB2701_00.jpg) -How many moles of B are present at 10 s?
-How many moles of B are present at 10 s?
A) 0.011
B) 0.220
C) 0.110
D) 0.014
E) 1.4 × 10-3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The concentration of S2O82- remaining at 400 s is ________ M.
A) +0.015
B) +0.035
C) -0.007
D) +0.045
E) +0.057
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is not a valid expression for the rate of the reaction below? 4NH3 + 7O2 → 4NO2 + 6H2O
A) - ![]()
![]()
B) ![]()
![]()
C) ![]()
![]()
D) - ![]()
![]()
E) All of the above are valid expressions of the reaction rate.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The half-life of a first-order reaction is 13 min. If the initial concentration of reactant is 0.085 M, it takes ________ min for it to decrease to 0.055 M.
A) 8.2
B) 11
C) 3.6
D) 0.048
E) 8.4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reaction: 3A → 2B The average rate of appearance of B is given by Δ[B]/Δt. Comparing the rate of appearance of B and the rate of disappearance of A, we get Δ[B]/Δt = ________ × (-Δ[A]/Δt) .
A) -2/3
B) +2/3
C) -3/2
D) +1
E) +3/2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
________ are used in automotive catalytic converters.
A) Heterogeneous catalysts
B) Homogeneous catalysts
C) Enzymes
D) Noble gases
E) Nonmetal oxides
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rate constant for a particular zero-order reaction is 0.075 M s-1. If the initial concentration of reactant is 0.537 M it takes ________ s for the concentration to decrease to 0.100 M.
A) 5.8
B) -5.8
C) -0.047
D) 7.2
E) 0.040
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At elevated temperatures, dinitrogen pentoxide decomposes to nitrogen dioxide and oxygen: 2N2O5(g) → 4NO2 (g) + O2 (g) When the rate of formation of NO2 is 5.5 × 10-4 M/s, the rate of decomposition of N2O5 is ________ M/s.
A) 2.2 × 10-3
B) 1.4 × 10-4
C) 10.1 × 10-4
D) 2.8 × 10-4
E) 5.5 × 10-4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction A (aq) → B (aq) is first order in [A]. A solution is prepared with [A] = 1.22 M. The following data are obtained as the reaction proceeds: ![The reaction A (aq) → B (aq) is first order in [A]. A solution is prepared with [A] = 1.22 M. The following data are obtained as the reaction proceeds: The rate constant for this reaction is ________ s<sup>-1</sup>. A) 0.23 B) 1.0 C) 0.17 D) 0.12 E) -0.12](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f62_14ad_a2ab_6f47494b9eef_TB2701_00.jpg) The rate constant for this reaction is ________ s-1.
The rate constant for this reaction is ________ s-1.
A) 0.23
B) 1.0
C) 0.17
D) 0.12
E) -0.12
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A reaction was found to be third order in A. Increasing the concentration of A by a factor of 3 will cause the reaction rate to ________.
A) remain constant
B) increase by a factor of 27
C) increase by a factor of 9
D) triple
E) decrease by a factor of the cube root of 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The combustion of ethylene proceeds by the reaction C2H4 (g) + 3O2 (g) → 2CO2 (g) + 2H2O (g) When the rate of disappearance of O2 is 0.13 M s-1, the rate of appearance of CO2 is ________ M s-1.
A) 0.087
B) 0.043
C) 0.39
D) 0.20
E) 0.26
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following, all are valid units for a reaction rate except ________.
A) mol/L
B) M/s
C) mol/hr
D) g/s
E) mol/L-hr
Correct Answer

verified
Correct Answer
verified
Short Answer
For a first-order reaction, a plot of ________ versus ________ is linear.
A)ln [A]t, ![For a first-order reaction, a plot of ________ versus ________ is linear. A)ln [A]<sub>t</sub>, B)ln [A]<sub>t</sub>, t C) , t D)[A]<sub>t</sub>, t E)t,](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_e97a_a2ab_df98b2796de5_TB2701_11.jpg) B)ln [A]t, t
C)
B)ln [A]t, t
C) ![For a first-order reaction, a plot of ________ versus ________ is linear. A)ln [A]<sub>t</sub>, B)ln [A]<sub>t</sub>, t C) , t D)[A]<sub>t</sub>, t E)t,](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f63_e97b_a2ab_1bdf2613abfc_TB2701_11.jpg) , t
D)[A]t, t
E)t,
, t
D)[A]t, t
E)t, ![For a first-order reaction, a plot of ________ versus ________ is linear. A)ln [A]<sub>t</sub>, B)ln [A]<sub>t</sub>, t C) , t D)[A]<sub>t</sub>, t E)t,](https://d2lvgg3v3hfg70.cloudfront.net/TB2701/11ea7cce_1f64_108c_a2ab_9bcdff11abad_TB2701_11.jpg)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At elevated temperatures, methylisonitrile (CH3NC) isomerizes to acetonitrile (CH3CN) : CH3NC (g) → CH3CN (g)
The dependence of the rate constant on temperature is studied and the graph below is prepared from the results. 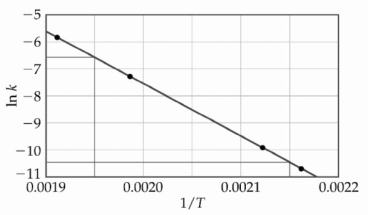 The energy of activation of this reaction is ________ kJ/mol.
The energy of activation of this reaction is ________ kJ/mol.
A) 160
B) 1.6 × 105
C) 4.4 × 10-7
D) 4.4 × 10-4
E) 1.9 × 104
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 134
Related Exams