A) 0.01 M H2SO3 (Ka = 1.4 × 10-2)
B) 0.01 M HCN (Ka = 6.2 × 10-10)
C) 0.01 M H2CO3 (Ka = 4.5 × 10-7)
D) 0.01 M HC3H5O2 (Ka = 1.3 × 10-5)
E) 0.01 M HOCl (Ka = 3.5 × 10-8)
Correct Answer

verified
Correct Answer
verified
True/False
A Lewis acid is an electron-pair acceptor, and a Lewis base is an electron-pair donor.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ka for HF is 7.0 × 10-4. Kb for the fluoride ion is ________.
A) 2.0 × 10-8
B) 1.4 × 10-11
C) 7.0 × 10-18
D) 7.0 × 10-4
E) 1.4 × 103
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is a Br∅nsted-Lowry base?
A) (CH3) 3N
B) CH3COOH
C) HF
D) HNO2
E) none of the above
Correct Answer

verified
Correct Answer
verified
Short Answer
A solution of formic acid is 3.0% dissociated at 25.0 °C. What is the original concentration (in M)of the formic acid solution? The Ka at 25.0 °C for formic acid is 1.8 × 10-4.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of an aqueous solution at 25.0 °C in which [OH-] is 0.0030 M?
A) 5.81
B) -11.48
C) 2.52
D) -2.52
E) 11.48
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the data in the table, which of the conjugate acids below is the weakest acid? 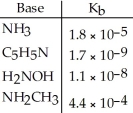
A) NH4+
B) C5H5NH+
C) H3NOH+
D) NH3CH3+
E) NH4+ and NH3CH3+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the concentration (in M) of hydronium ions in a solution at 25.0 °C with pH = 4.146?
A) 4.15
B) 9.85
C) 1.40 × 10-10
D) 7.15 × 10-5
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following acids will be the strongest?
A) H2SO4
B) HSO4-
C) H2SO3
D) H2SeO4
E) HSO3-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molar concentration of hydroxide ion in pure water at 25 °C is ________.
A) 1.00
B) 0.00
C) 1.0 × 10-14
D) 1.0 × 10-7
E) 7.00
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the data in the table, which of the conjugate bases below is the strongest base? 
A) OAc-
B) C7H5O2-
C) NO2-
D) F-
E) OAc- and C7H5O2-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A Br∅nsted-Lowry acid is defined as a substance that ________.
A) increases Ka when placed in H2O
B) decreases [H+] when placed in H2O
C) increases [OH-] when placed in H2O
D) acts as a proton acceptor
E) acts as a proton donor
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In acidic solution, ________.
A) [H3O+] > [OH-]
B) [H3O+] = [OH-]
C) [H3O+] < [OH-]
D) [OH-] > 7.00
E) [H3O+] = 0M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is a Br∅nsted-Lowry acid?
A) (CH3) 3NH+
B) CH3COOH
C) HF
D) HNO2
E) all of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Ka of hypochlorous acid (HClO) is 3.00 × 10-8. What is the pH at 25.0 °C of an aqueous solution that is 0.0200 M in HClO?
A) +2.45
B) -2.45
C) -9.22
D) +9.22
E) +4.61
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of an aqueous solution at 25.0 °C that contains 3.98 × 10-9 M hydroxide ion?
A) 8.40
B) 5.60
C) 9.00
D) 3.98
E) 7.00
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Classify the following compounds as weak bases (W) or strong bases (S) : methylamine carbonate ion potassium hydroxide
A) W W S
B) S S S
C) S W W
D) W S S
E) W S W
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the pH of 0.726 M anilinium hydrochloride (C6H5NH3Cl) solution in water, given that Kb for aniline is 3.83 × 10-4.
A) 1.78
B) 12.22
C) 5.36
D) 8.64
E) 12.42
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Ka for formic acid (HCO2H) is 1.8 × 10-4. What is the pH of a 0.20 M aqueous solution of sodium formate (NaHCO2) ?
A) 11.64
B) 5.48
C) 3.39
D) 8.52
E) 4.26
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which solution below has the highest concentration of hydronium ions?
A) pH = 3.0
B) pH = 10
C) pH = 7.0
D) pH = 6.4
E) pH = 11.2
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 139
Related Exams