A) electrons
B) protons, neutrons, and electrons
C) protons and neutrons
D) protons and electrons
E) protons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Silver has two naturally occurring isotopes with the following isotopic masses: 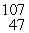 Ar
Ar 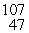 Ar 106.90509 108.9047
The average atomic mass of silver is 107.8682 amu. The fractional abundance of the lighter of the two isotopes is __________.
Ar 106.90509 108.9047
The average atomic mass of silver is 107.8682 amu. The fractional abundance of the lighter of the two isotopes is __________.
A) 0.24221
B) 0.48168
C) 0.51835
D) 0.75783
E) 0.90474
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An unknown element is found to have three naturally occurring isotopes with atomic masses of 35.9675 (0.337%) , 37.9627 (0.063%) , and 39.9624 (99.600%) . Which of the following is the unknown element?
A) Ar
B) K
C) Cl
D) Ca
E) None of the above could be the unknown element.
Correct Answer

verified
Correct Answer
verified
True/False
H2SeO4 is called selenic acid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is the formula of hydrochloric acid?
A) HClO3
B) HClO4
C) HClO
D) HCl
E) HClO2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Elements in Group 1A are known as the __________.
A) chalcogens
B) alkaline earth metals
C) alkali metals
D) halogens
E) noble gases
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Gravitational forces act between objects in proportion to their __________.
A) volumes
B) masses
C) charges
D) polarizability
E) densities
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct name for HBrO4 is __________.
A) hydrobromic acid
B) perbromic acid
C) bromic acid
D) bromous acid
E) hydrobromous acid
Correct Answer

verified
Correct Answer
verified
True/False
The formula for chromium (II)iodide is CrI2.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which isotope has 36 electrons in an atom?
A) ![]() Kr
Kr
B) ![]() Br
Br
C) ![]() Se
Se
D) ![]() Cl
Cl
E) ![]() Hg
Hg
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Elements in the same group of the periodic table typically have __________.
A) similar mass numbers
B) similar physical properties only
C) similar chemical properties only
D) similar atomic masses
E) similar physical and chemical properties
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Copper and chlorine form an ionic compound whose formula is CuCl2. The name of this compound is __________.
A) copper chlorine
B) copper (III) dichloride
C) monocopper dichloride
D) copper (II) dichloride
E) cupric chloride
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Vertical columns of the periodic table are known as __________.
A) metals
B) periods
C) nonmetals
D) groups
E) metalloids
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom of 118Xe contains __________ neutrons.
A) 54
B) 172
C) 64
D) 110
E) 118
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When calcium reacts with sulfur the compound formed is __________.
A) Ca2S2
B) Ca3S2
C) CaS2
D) CaS2
E) Ca2S3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The name of the ionic compound V2O3 is __________.
A) vanadium(III) oxide
B) vanadium oxide
C) vanadium(II) oxide
D) vanadium(III) trioxide
E) divanadium trioxide
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct name for MgF2 is __________.
A) monomagnesium difluoride
B) magnesium difluoride
C) manganese difluoride
D) manganese bifluoride
E) magnesium fluoride
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mass number of an atom of 128Xe is __________.
A) 54
B) 182
C) 74
D) 128
E) 120
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The element X has three naturally occurring isotopes. The isotopic masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is __________ amu. 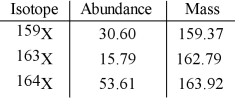
A) 161.75
B) 162.03
C) 162.35
D) 163.15
E) 33.33
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The name of PCl3 is __________.
A) potassium chloride
B) phosphorus trichloride
C) phosphorous(III) chloride
D) monophosphorous trichloride
E) trichloro potassium
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 230
Related Exams