A) bent
B) trigonal planar
C) trigonal pyramidal
D) tetrahedral
E) T-shaped
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molecular geometry consists of __________. (a) a nonbonding pair of electrons (b) a single bond (c) a multiple bond
A) a only
B) b only
C) c only
D) a, b, and c
E) b and c
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Valence bond theory does not address the issue of __________.
A) excited states of molecules
B) molecular shape
C) covalent bonding
D) hybridization
E) multiple bonds
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules or ions will exhibit delocalized bonding? SO2 SO3 SO32-
A) SO2, SO3, and SO32-
B) SO32- only
C) SO2 and SO3
D) SO3 and SO32-
E) None of the above will exhibit delocalized bonding.
Correct Answer

verified
Correct Answer
verified
True/False
Boron trifluoride has three bonding domains and its electron domain geometry is trigonal planar.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molecular geometry of the CHF3 molecule is __________, and the molecule is __________.
A) trigonal pyramidal, polar
B) tetrahedral, nonpolar
C) seesaw, nonpolar
D) tetrahedral, polar
E) seesaw, polar
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the molecules below, only __________ is nonpolar.
A) BF3
B) NF3
C) IF3
D) PBr3
E) BrCl3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A triatomic molecule cannot be linear if the hybridization of the central atoms is __________.
A) sp
B) sp2
C) sp3
D) sp2 or sp3
E) sp2d or sp3d2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following, the central atom is sp3d2 hybridized only in __________.
A) PCl5
B) XeF4
C) PH3
D) Br3-
E) BeF2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The O-S-O bond angle in SO2 is slightly less than __________.
A) 90°
B) 109.5°
C) 120°
D) 180°
E) 60°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For molecules of the general formula ABn, n can be greater than four __________.
A) for any element A
B) only when A is an element from the third period or below the third period
C) only when A is boron or beryllium
D) only when A is carbon
E) only when A is Xe
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on molecular orbital theory, the bond order of the C-C bond in the C2 molecule is __________.
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Short Answer
The more unpaired electrons in a species, the stronger is the force of magnetic attraction. This is called __________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the molecules does the central atom utilize d orbitals to form hybrid orbitals?
A) (i) and (ii)
B) (iii) only
C) (i) and (v)
D) (iii) , (iv) , and (v)
E) (v) only
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The electron domain and molecular geometry of BrO2- is __________.
A) tetrahedral, trigonal planar
B) trigonal planar, trigonal planar
C) trigonal pyramidal, linear
D) tetrahedral, bent
E) trigonal pyramidal, seesaw
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following, __________ appear(s) to gain mass in a magnetic field. B2 N2 O2
A) O2 only
B) N2 only
C) B2 and N2
D) N2 and O2
E) B2 and O2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The electron-domain geometry of a carbon-centered compound is tetrahedral. The hybridization of the central carbon atom is __________.
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molecular geometry of the right-most carbon in the molecule below is __________. 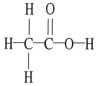
A) trigonal planar
B) trigonal bipyramidal
C) tetrahedral
D) octahedral
E) T-shaped
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following, only __________ has sp2 hybridization of the central atom.
A) PH3
B) CO32-
C) ICl3
D) l3-
E) PF5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In order to exhibit delocalized π bonding, a molecule must have __________.
A) at least two π bonds
B) at least two resonance structures
C) at least three σ bonds
D) at least four atoms
E) trigonal planar electron domain geometry
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 177
Related Exams