A) 1-chloro-2-methylcyclohexane
B) 1-methyl-2-chlorocyclohexane
C) 1-chloro-5-methylcyclohexane
D) 1-methyl-5-chlorocyclohexane
E) 1, 2-chloromethylcyclohexane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is diisopropyl ether?
A) CH3CH2CH2-O-CH2CH2CH3
B) ![]()
C) CH3CH2CH2OCH(CH3) 2
D) (CH3) 3COC(CH3) 3
E) (CH3) 2CHOCH(CH3) 2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the least stable conformation for 1-tert-butyl-3-methylcyclohexane.
A) tert-butyl is axial and the methyl is equatorial.
B) tert-butyl is axial and the methyl is axial.
C) tert-butyl is equatorial and the methyl is axial.
D) tert-butyl is equatorial and the methyl is equatorial.
E) All are equally stable.
Correct Answer

verified
Correct Answer
verified
Short Answer
Provide the IUPAC name of the compound. H2NCH2CH2CH2CH2OH
Correct Answer

verified
Correct Answer
verified
Essay
Give structures for the three isomers with molecular formula C5H12 and provide the common name of each.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If an acyclic alkane hydrocarbon contains n carbon atoms, how many hydrogen atoms must it also contain?
A) n
B) n + 2
C) n - 2
D) 2n
E) 2n + 2
Correct Answer

verified
Correct Answer
verified
Short Answer
Provide an acceptable name for the alkane shown below. 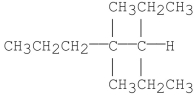
Correct Answer

verified
3-ethyl-4,...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following describes the most stable conformation of trans-1-tert-butyl-3-methylcyclohexane?
A) Both groups are equatorial.
B) Both groups are axial.
C) The tert-butyl group is equatorial and the methyl group is axial.
D) The tert-butyl group is axial and the methyl group is equatorial.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Essay
Draw an acceptable structure for sec-butylcyclopentane.
Correct Answer

verified
Correct Answer
verified
Essay
Draw the Newman projection that represents the most stable conformation of 3,3-dimethylhexane viewed along the C3-C4 bond.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the number of secondary hydrogens in the following structure. 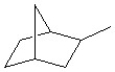
A) 4
B) 6
C) 8
D) 10
E) 12
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the number of hydrogens in limonene. 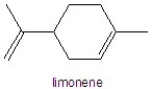
A) 8
B) 10
C) 12
D) 16
E) 18
Correct Answer

verified
Correct Answer
verified
Essay
Draw a Newman projection of the most stable conformation of 2-methylpropane.
Correct Answer

verified
Correct Answer
verified
Essay
Draw all ethers with molecular formula C4H10O.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following has the greatest van der Waal's interaction between molecules of the same kind?
A) ![]()
B) CH3CH2CH2CH3
C) ![]()
D) CH3CH2CH2CH2CH3
E) ![]()
Correct Answer

verified
Correct Answer
verified
Essay
Draw the most stable conformer of cis-1-ethyl-4-methylcyclohexane.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the compounds below will form hydrogen bonds between its molecules?
A) CH3CH2CH2F
B) CH3CH2CH2CH3
C) (CH3) 3N
D) CH3CH2OCH3
E) CH3NHCH2CH3
Correct Answer

verified
Correct Answer
verified
Essay
Draw an acceptable structure for 6-ethyl-2,6, 7-trimethyl-5-propylnonane.
Correct Answer

verified
Correct Answer
verified
Essay
Would you expect sodium chloride (NaCl)to be highly soluble in the organic solvent hexane (CH3CH2CH2CH2CH2CH3)? Briefly explain your answer.
Correct Answer

verified
One would not expect NaCl to be highly s...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Give the IUPAC name for the following structure: 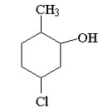
A) 3-chloro-6-methylcyclohexanol
B) 2-methyl-5-chlorocyclohexanol
C) 1-chloro-4-methylcyclohexanol
D) 5-chloro-2-methylcyclohexanol
E) 2-methyl-3-chlorocyclohexanol
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 123
Related Exams