A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many proton NMR singlets will 2-bromo-3-methyl-2-butene exhibit?
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer

verified
Correct Answer
verified
Essay
Deduce the identity of the following compound from the 1H NMR spectral data given. C3H6Br2 : two peaks: a 2H quintet and a 4H triplet
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound has a 1H NMR spectrum consisting of the following peaks: 0.9 (6H, d) , 1.0 (3H, t) , 2.2 (2H, q) , and 4.0 (1H, septet) ?
A) (CH3) 2CHCH2O2CCH3
B) (CH3) 2CHCH2CO2CH3
C) (CH3) 2CHO2CCH2CH3
D) (CH3) 2CHCO2CH2CH3
E) (CH3) 2CHOCH2CH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the structure of a compound that has a formula of C8H11N and has signals in the 13C NMR spectrum at 25.9 ppm (CH3) , 51.1 ppm (CH) , 125.9 ppm (CH) , 126.6 ppm (CH) , 128.3 ppm (CH) , and 148.5 ppm (C) .
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Essay
Give one reason why 13C NMR is less sensitive than 1H NMR.
Correct Answer

verified
Natural isotopic abundance of ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
How many signals would you expect to see in the 1H NMR spectrum of the following compound? 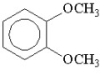
A) 5
B) 4
C) 2
D) 3
E) 1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following electromagnetic wave types is used in nuclear magnetic resonance spectroscopy?
A) X-ray
B) infrared
C) visible
D) radio
E) ultraviolet
Correct Answer

verified
Correct Answer
verified
Essay
Deduce the identity of the following compound from the spectral data given. C5H10O: 1H NMR, δ 1.2 (6H, doublet), 2.1 (3H, singlet), 2.8 (1H, septet); IR, 2980, 1710 cm-1; MS, m/z 71,
Correct Answer

verified
Correct Answer
verified
Essay
What information does a HETCOR spectrum give?
Correct Answer

verified
Coupling between hydrogens and the hydrogens to which they are attached to.
Correct Answer
verified
Multiple Choice
If a chemical shift of an NMR signal is 7.2 ppm measured in a 60 MHz NMR spectrometer, how many Hz would this signal be from the TMS signal?
A) 8) 3 Hz
B) 432 Hz
C) 0) 12 Hz
D) 72 Hz
E) 60 Hz
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many signals would you expect to see in the 1H NMR spectrum of the following compound? 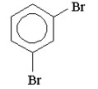
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What splitting pattern is observed in the proton NMR spectrum for the indicated hydrogens? 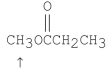
A) singlet
B) doublet
C) triplet
D) quartet
E) septet
Correct Answer

verified
A
Correct Answer
verified
Essay
Why is Fourier transform NMR spectroscopy preferred over continuous wave as a technique for 13C NMR?
Correct Answer

verified
13C nuclei have a low sensitivity which re...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What splitting pattern is observed in the proton NMR spectrum for the indicated hydrogens? CH3OCH2CH2OCH3 ↑
A) singlet
B) doublet
C) triplet
D) quartet
E) septet
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
How many signals would you expect to see in the 1H NMR spectrum of the following compound? ClCH2CH2Cl
A) 5
B) 4
C) 3
D) 2
E) 1
Correct Answer

verified
Correct Answer
verified
Short Answer
How many distinct carbon signals are expected in the proton-decoupled 13C NMR spectrum of the compound below? 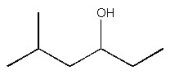
Correct Answer

verified
Correct Answer
verified
Essay
Deduce the identity of the following compound from the 1H NMR spectral data given. C8H18O : δ 0.89 (6H, doublet), 1.87 (1H, multiplet), 3.17 (2H, doublet)(ppm)
Correct Answer

verified
Correct Answer
verified
Essay
An unknown compound, C4H8Br2, gave the following proton NMR data: Singlet at 1.97 ppm (6H) Singlet at 3.89 ppm (2H) What is the compound?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the structure of a compound that has a formula of C9H10O2 and has signals in the 13C NMR spectrum at 13.6 ppm (CH3) ; 59.1 ppm (CH2) ; 128.4 ppm (CH) ; 129.7 ppm (CH) ; 130.5 ppm (C) ; 132.8 ppm (CH) ; and167.0 ppm (C) .
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 110
Related Exams