A) Ca
B) As
C) Mn
D) Ni
Correct Answer

verified
Correct Answer
verified
Short Answer
The smallest number that can be used for m or n in the Balmer-Rydberg equation is ________ and the largest number is ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is true?
A) The Bohr atom is the model currently accepted for electrons in atoms.
B) Electrons travel around the nucleus in circular orbits.
C) There is a 5% chance of finding an electron in an atom outside its orbital.
D) The square of the wave function gives the probability of finding the electron.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the ground-state electron configuration of tellurium?
A) [Kr]4d105s25p4
B) [Kr]5s25p65d8
C) [Kr]5s25p4
D) [Kr]4f144d105s25p4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the ground-state valence-shell electron configuration of the group of elements indicated by the shaded portion of the periodic table? 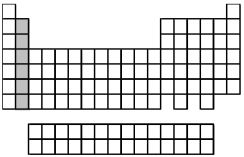
A) ns2
B) ns2np2
C) ns2(n-1) d2
D) ns2(n-2) f2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The absorption of light of frequency 1.16 × 1011 Hz is required for CO molecules to go from the lowest rotational energy level to the next highest rotational energy level.Determine the energy for this transition in kJ/mol.(h = 6.626 × 10-34 J ∙ s)
A) 7.69 × 10-23 kJ/mol
B) 0.0463 kJ/mol
C) 46.3 kJ/mol
D) 949 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not quantized?
A) the charge on a monatomic ion
B) the distance between two objects
C) the population of the United States
D) the static charge on a balloon rubbed with wool
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many orbitals are there in the sixth shell?
A) 5
B) 6
C) 15
D) 36
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a valid set of quantum numbers?
A) n = 2,l = 1,ml = 0,and ms = +1/2
B) n = 2,l = 1,ml = -1,and ms = +1/2
C) n = 3,l = 0,ml = 0,and ms = +1/2
D) n = 3,l = 2,ml = 3,and ms = +1/2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Two electromagnetic waves are represented below. 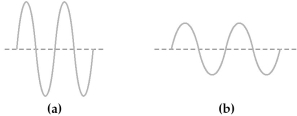 -Wave (a) has the
-Wave (a) has the
A) longer wavelength and higher frequency than wave (b) .
B) longer wavelength and lower frequency than wave (b) .
C) shorter wavelength and higher frequency than wave (b) .
D) shorter wavelength and lower frequency than wave (b) .
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Light can be made to have a higher intensity by raising its
A) amplitude.
B) energy.
C) frequency.
D) wavelength.
Correct Answer

verified
Correct Answer
verified
Short Answer
A solution to the Schrödinger wave equation is a ________,or orbital,represented by the symbol Ψ,and the probability of finding an electron defined by Ψ within a given volume of space around the nucleus is ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is true? The probability density
A) for all s orbitals is independent of direction from the nucleus.
B) for all s orbitals is independent of distance from the nucleus.
C) is independent of direction from the nucleus for 1s orbitals only.
D) is independent of distance from the nucleus for 1s orbitals only.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Photochemists use electromagnetic radiation to initiate chemical reactions,often by providing the energy required to break bonds within a molecule.Lowering which of the following will result in electromagnetic radiation having more energy per photon?
A) amplitude
B) frequency
C) intensity
D) wavelength
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Arrange the following spectral regions in order of increasing energy: infrared,microwave,ultraviolet,visible.
A) microwave < infrared < visible < ultraviolet
B) microwave < visible < infrared < ultraviolet
C) ultraviolet < infrared < visible < microwave
D) ultraviolet < visible < infrared < microwave
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The spheres below represent atoms of Sb,As,P,and N (not necessarily in that order) . 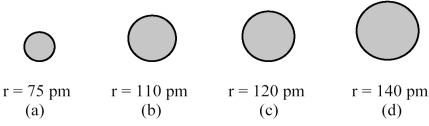 -Which one of these spheres represents an atom of As?
-Which one of these spheres represents an atom of As?
A) sphere (a)
B) sphere (b)
C) sphere (c)
D) sphere (d)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The spheres below represent atoms of Li,Be,B,and F (not necessarily in that order) . 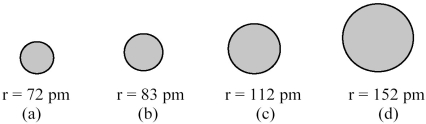 -Which one of these spheres represents an atom of F?
-Which one of these spheres represents an atom of F?
A) sphere (a)
B) sphere (b)
C) sphere (c)
D) sphere (d)
Correct Answer

verified
Correct Answer
verified
Short Answer
An orbital that as the appearance of a three-dimensional clover leaf has a quantum number l = ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many valence electrons does a neutral polonium atom have?
A) 2
B) 4
C) 6
D) 84
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An element in a ground state electron configuration has 4 electrons in the 4p orbitals.Which of the following statements can not describe the electron configurations in this atom?
A) At least one electron has an orbital angular momentum (l) of 2.
B) Six electrons are in the n=4 shell.
C) The valence electron configuration is identical to carbon.
D) No electrons have an orbital angular momentum (l) of 3.3.
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 168
Related Exams