A) 2-propanal.
B) 3-propanal.
C) 2-propanone.
D) 1-propanone.
E) dimethyl ketone.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a hemiacetal?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the compound shown? 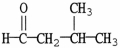
A) 2-methyl-4-butanone
B) 2-methyl-1-butanone
C) 2-methylbutanal
D) 3-methylbutanal
E) isopentanal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following products is formed when hydrogen is reacted with 3-methyl-2-butanone?
A) a primary alcohol
B) a secondary alcohol
C) a tertiary alcohol
D) an acetal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The carbonyl group is
A) a general term for any functional group involving a carbon-oxygen bond.
B) found only in aldehydes and ketones.
C) a functional group in which carbon and oxygen are joined by a double bond.
D) a functional group with a 6-membered ring where at least one atom is oxygen.
E) produced by reduction reactions of primary or secondary alcohols.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule is an aldehyde?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which molecule is acetone?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A compound with an -OH group and an ether-like -OR group bonded to the same carbon atom is
A) an acetal.
B) an aldol.
C) a diol.
D) a hemiacetal.
E) a simple ether.
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
All of the following statements about oxidation of carbonyls are true except
A) the Tollens' test involves oxidation of Ag+.
B) the Benedict's test involves reduction of Cu2+.
C) oxidation of aldehydes produces carboxylic acids.
D) ketones do not react with mild oxidizing agents.
E) All of the statements are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following. -an example of an acetal
A) ![]()
B) CH3CH2COCH2CH3
C) CH3CH2CH2 H2CHO
D) the simplest aldehyde,also named formaldehyde
E) the simplest ketone,also named acetone
F) a functional group consisting of a carbon atom with a double bond to an oxygen atom
G) a functional group consisting of a carbonyl group bonded to to a carbon that is bonded
to one hydrogen atom and two other carbon atoms.
H) a compound with a carbon atom that is bonded to both an alcohol-like group and an ether-like group.
I) a functional group consisting of a carbonyl group bonded to two other carbon atoms
J) a compound with a carbon atom that is bonded to two ether-like groups.
K) ![]()
L) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All of the following are true concerning a two-carbon aldehyde except
A) its condensed formula is C![]()
CHO.
B) its systematic name is ethanal.
C) its common name is acetaldehyde.
D) it has a higher boiling point than an alcohol of similar molecular weight.
E) its structural formula is:![]()
.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Oxidation of an aldehyde produces a
A) carboxylic acid.
B) primary alcohol.
C) secondary alcohol.
D) tertiary alcohol.
E) ketone.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following. -ketone
A) ![]()
B) CH3CH2COCH2CH3
C) CH3CH2CH2 H2CHO
D) the simplest aldehyde,also named formaldehyde
E) the simplest ketone,also named acetone
F) a functional group consisting of a carbon atom with a double bond to an oxygen atom
G) a functional group consisting of a carbonyl group bonded to to a carbon that is bonded
to one hydrogen atom and two other carbon atoms.
H) a compound with a carbon atom that is bonded to both an alcohol-like group and an ether-like group.
I) a functional group consisting of a carbonyl group bonded to two other carbon atoms
J) a compound with a carbon atom that is bonded to two ether-like groups.
K) ![]()
L) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One of the major differences between aldehydes and ketones as compared to other carbonyl compounds is that in aldehydes and ketones
A) the molar masses tend to be much smaller than in the other types of compounds.
B) the carbonyl carbon has bond angles of 120°C,unlike the comparable bond angles in other carbonyl compounds.
C) the polar carbon-oxygen bond is less reactive than the hydrocarbon portion of the molecule.
D) the carbonyl group carbon atom is bonded to atoms that do not attract electrons strongly.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which observation denotes a positive Tollens' test?
A) The light blue color of the reagent disappears.
B) A brick-red precipitate forms.
C) A silver deposit forms on the glass surface.
D) A silver wire dissolves.
E) Bubbles of oxygen gas are produced.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name for diisopropyl ketone?
A) 2,2-dimethyl-3-pentanone
B) 2-dimethyl-3-pentanone
C) 2,4-dimethyl-3-pentanone
D) 2,4-dimethyl-3-propanone
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All of the following are true concerning a three-carbon ketone except
A) its condensed formula is CH3COCH3.
B) its systematic name is propanone.
C) its common name is acetone.
D) another acceptable name is methyl ethyl ketone.
E) its structural formula is![]()
.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All of the statements concerning the carbonyl group in aldehydes and ketones are true except
A) the bond is polar,with a slight negative charge on the oxygen atom.
B) the bond angles about the central carbon atom are 120°.
C) the carbonyl group is planar.
D) in condensed form the carbonyl group can be written as -CHO.
E) because the bond is polar,carbonyl groups readily form hydrogen bonds with each other.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound has the lowest boiling point?
A) CH3CHO
B) CH3CH2CHO
C) CH3CH2OH
D) CH3CH2CH2OH
E) CH3COCH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All of the following are properties of acetone except
A) volatility.
B) flammability.
C) intoxication.
D) solvent for organic substances.
E) nutrient.
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 77
Related Exams