A) iodine.
B) oxygen.
C) argon.
D) potassium.
E) sulfur.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which column of the periodic table contains only nonmetals?
A) 4A
B) 5A
C) 6A
D) 7A
E) 3A
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The value of Z for the element 37Ar is
A) 37.
B) 39.945.
C) 19.
D) 21.945.
E) 18.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which group contains only f-block elements?
A) Ni,Pd,Pt
B) Si,Ge,As
C) Ce,Pr,Nd
D) Kr,Xe,Rn
E) Po,Fr,Ac
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represents a pair of isotopes?
A) ![]() C,
C, ![]() N
N
B) ![]() H,
H, ![]() H
H
C) ![]() S,
S,![]() S2
S2
D) O2,O3
E) all are pairs of isotopes
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Cobalt is element 27.Cobalt-60 is used in the medical treatment of cancer.How many neutrons and protons are contained in the nucleus of this isotope?
A) 27 neutrons,33 protons
B) 33 neutrons,27 protons
C) 27 neutrons,27 protons
D) 33 neutrons,33 protons
E) 60 neutrons,27 protons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The number of valence electrons in a main group element is given by
A) atomic number.
B) atomic weight.
C) group number.
D) mass number.
E) none of the above.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which group contains only d-block elements?
A) Ni,Pd,Pt
B) Si,Ge,As
C) Ce,Pr,Nd
D) Kr,Xe,Rn
E) Po,Fr,Ac
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the electron configuration of Fe?
A) 1s2 2s2 2p6 3s2 3p6 3d8
B) 1s2 2s2 2p6 3s2 3p6 4s2 3d6
C) 1s2 2s2 2p6 3s2 3p6 4s2 4p6
D) 1s2 2s2 2p6 3s2 3p6 4s2
E) 1s2 2s2 2p6 3s2 3p6 3d6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Another name for atomic mass unit (amu) is the
A) avogadro.
B) dalton.
C) Kekule
D) kelvin.
E) mendeleev.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The number of neutrons in an atom is equal to
A) atomic number - mass number.
B) mass number - atomic number.
C) the atomic number.
D) the mass number.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The number of valence electrons in an element with electron configuration 1s2 2s2 2p6 3s2 3p4 is
A) 2.
B) 4.
C) 6.
D) 8.
E) 16.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Valence electrons in the main group elements are contained in which type(s) of orbitals?
A) s
B) p
C) s and p
D) d
E) f
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which group contains only p-block elements?
A) N,S,Br
B) Mn,Cu,Ag
C) K,Mg,Al
D) Ce,Pr,Nd
E) Na,P,Cl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many neutrons does an atom of 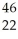 Ti have?
Ti have?
A) 0
B) 22
C) 24
D) 46
E) 68
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What fourth period element is represented by the dot structure shown? X:
A) K
B) Ca
C) V
D) As
E) Ti
Correct Answer

verified
Correct Answer
verified
Showing 81 - 96 of 96
Related Exams