Correct Answer

verified
In most molecules,the Lewis dot structur...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which property could describe a covalent compound?
A) It conducts electricity when melted.
B) It is a gas at room temperature.
C) It is composed of a non-metal and a metal.
D) It conducts electricity when dissolved in water.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A chemical bond formed when two atoms share six electrons is a ________ bond;it is best described as ________.
A) double;covalent
B) double;ionic
C) single;covalent
D) single;ionic
E) triple;covalent
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The covalent bonding model is most useful in describing which of the following compounds?
A) CoCl2
B) MgCl2
C) NiCl2
D) SCl2
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following. -sulfur dioxide
A) ICl
B) C ![]()
C) N ![]()
D) PC ![]()
E) ![]()
![]()
F) CO
G) S ![]()
H) S ![]()
I) O ![]()
J) Br ![]()
K) CC ![]()
L) I ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many double bonds are there in a molecule of SF2?
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
True/False
Covalent compounds form distinct molecules,and therefore may exist as gases,liquids,or solids at room temperature,depending on the characteristics of the compound.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following. -carbon monoxide
A) ICl
B) C ![]()
C) N ![]()
D) PC ![]()
E) ![]()
![]()
F) CO
G) S ![]()
H) S ![]()
I) O ![]()
J) Br ![]()
K) CC ![]()
L) I ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element listed is the least electronegative?
A) nitrogen
B) hydrogen
C) oxygen
D) fluorine
E) chlorine
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A chemical bond formed between two identical atoms is a(an) ________ bond.
A) atomic
B) covalent
C) hydrogen
D) ionic
E) molecular
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule in which the central atom forms three single bonds and has one lone pair is said to have a ________ shape.
A) bent
B) linear
C) planar
D) pyramidal
E) tetrahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formula for phosphorus pentafluoride is
A) PhF5.
B) PF5.
C) P5F.
D) (PF) 5.
E) P5F5.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule in which the central atom forms one double bond and two single bonds is said to have a ________ shape.
A) bent
B) linear
C) trigonal planar
D) pyramidal
E) tetrahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has a CCH bond angle of 180°?
A) HCCH
B) H2CCH2
C) H3CCH3
D) H3CCHO
E) none of them
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A section of the Periodic Table containing main group elements is shown.Which bond would be least polar? 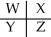
A) WX
B) YZ
C) WZ
D) WY
E) cannot be determined without more specific information
Correct Answer

verified
Correct Answer
verified
Essay
Draw the Lewis structure for CCl4.What is the molecular geometry of this compound? Is the molecule polar or nonpolar?
Correct Answer

verified
 tetrahedr...
tetrahedr...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
A chemical bond formed when two atoms share two pairs of electrons is a ________ bond;it is best described as ________.
A) double;covalent
B) double;ionic
C) single;covalent
D) single;ionic
E) triple;covalent
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The VSEPR model of molecular structure requires a knowledge of ________ to predict the geometry of an atom in a molecule.
A) the number of atoms bonded to the atom of interest
B) the total number of atoms in the molecule
C) the number of electron pairs on the atom of interest
D) both A and C
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The water molecule has a ________ geometry because its central atom has ________ bonds and ________ lone pairs of electrons.
A) bent;two;two
B) linear;two;two
C) pyramidal;three;one
D) tetrahedral;four;zero
E) planar triangular;three;one
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A chemical bond formed when two atoms share three pairs of electrons is a ________ bond;it is best described as ________.
A) double;covalent
B) single;covalent
C) triple;covalent
D) double;ionic
E) triple;ionic
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 78
Related Exams