A) 63.07 amu
B) 118.13 amu
C) 132.13 amu
D) 128.11 amu
E) 114.10 amu
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Interpret in words the equation shown. P4O10(s) + 6 H2O(l) → 4 H3PO4(aq)
A) One mole of solid tetraphosphorus decaoxide reacts with six moles of liquid water to produce four moles of phosphoric acid solution.
B) Zero moles of solid phosphoric oxide dissolve in six moles of water to produce four moles of phosphorous acid.
C) One mole of phosphorus(X) oxide combines with six moles of water to produce four moles of solution of hydrogen phosphate.
D) Four moles of solid phosphorus,five moles of diatomic oxygen gas,and six moles of liquid water react together to produce four moles of phosphoric acid in solution.
E) Four atoms of phosphorus,16 atoms of oxygen,and 12 atoms of hydrogen rearrange to produce four molecules of phosphoric acid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a reaction to produce ammonia,the theoretical yield is 420.g.What is the percent yield if the actual yield is 350.g?
A) 20.0%
B) 83.3%
C) 16.7%
D) 120%
E) 105%
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What does the balanced equation given below mean? CH4 + 2 O2 → CO2 + 2H2O
A) One mole of methane reacts with two moles of oxygen to produce one mole of carbon dioxide and two moles of water.
B) One gram of methane reacts with two grams of oxygen to produce one gram of carbon dioxide and two grams of water.
C) One molecule of methane reacts with two molecules of oxygen to produce one molecule of carbon dioxide and two molecules of water.
D) A,B,and C are correct.
E) A and C are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formula mass of copper(II) chloride is ________ g.
A) 98.90
B) 133.00
C) 134.45
D) 162.53
E) 197.80
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the reaction shown,what is the mole ratio that would be used to determine the number of moles of oxygen needed to react with 3.2 moles of C4H10? 2 C4H10+ 13O2→ 8 CO2+ 10 H2O
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following equation represents the formation of nitrogen dioxide,a major component of smog, 2 NO + O2 → 2 NO2 If 0.68 mole of NO is reacted with 0.79 mole of O2 to produce NO2,the limiting reactant is
A) both NO and O2.
B) NO.
C) O2.
D) NO2.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How much Ca(NO3) 2 should be weighed out to have 0.650 mol?
A) 66.4 g
B) 97.6 g
C) 107 g
D) 133 g
E) 165 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement based on the mole concept is not correct?
A) The molar mass of a diatomic element is its atomic weight in grams times two.
B) The number of atoms in one mole of platinum is the same as the number of atoms in one mole of uranium.
C) One mole of sodium chloride,NaCl,contains the same number of ions as one mole of calcium sulfate,CaSO4.
D) One mole of methane,CH4,contains the same number of atoms as one mole of carbon dioxide,CO2.
E) One mole of bromine,Br2,contains the same number of molecules as one mole of propane,C3H8.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement concerning the mole is not correct?
A) The molar mass of any compound is equal to its molecular weight in grams.
B) A mole of metal contains NA atoms of that metal.
C) A mole of any compound contains one mole of each kind of atom found in that compound.
D) A mole of a diatomic element contains 2 × NA moles of atoms of the element.
E) All of these statements are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following.
-The molar mass of BaC 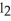
A) 212.4 g
B) 219 g
C) 225 g
D) 208.2 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Acid rain forms in the upper atmosphere by the reaction of sulfur trioxide with water forming sulfuric acid.Calculate the mass in grams of 5.65 × 1021 molecules of SO3.
A) 2.72 × 1047 g
B) 1.17 × 10-4 g
C) 0.751 g
D) 0.0751 g
E) 1.17 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The combustion of propane gas (C3H8) is used to fuel barbeque grills.In order to produce 5.65 moles of water how many moles of oxygen gas are needed? C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(l)
A) 3.39 mol
B) 4.52 mol
C) 7.06 mol
D) 1.13 mol
E) 1.41 mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a tablet of acetaminophen,C8H9NO2,weighs 324 mg,how many moles are there in the tablet?
A) 2.15 × 10-3
B) 48.9
C) 4.89 × 104
D) 2.15
E) 466
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formula weight of Al2(SO4) 3 is ________ grams.
A) 214.14
B) 278.02
C) 315.14
D) 342.14
E) 450.14
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the limiting reactant when 3.00 g of calcium are reacted with 8.00 g of water to produce hydrogen gas in the following equation? Ca(s) + 2 H2O(l) → Ca(OH) 2(aq) + H2(g)
A) Ca
B) H2O
C) Ca(OH) 2
D) H2
E) not enough information is given
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of NaHCO3 are present in a 2.00 g sample?
A) 2.38 × 10-2 mol
B) 1.27 × 10-2 mol
C) 2.00 mol
D) 85.0 mol
E) 168 mol
Correct Answer

verified
Correct Answer
verified
Showing 41 - 57 of 57
Related Exams