A) π*2s
B) π2s
C) σ*1p
D) π*2p
E) π2p
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the hybrid orbitals used for bonding by Xe in a XeCl4 molecule?
A) sp2
B) sp3d2
C) sp3
D) sp3d
E) sp
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a C=C bond,the σ bond results from overlap of ________ orbitals and the π bond(s) result from overlap of ________ orbitals.
A) sp2-hybrid, p-atomic
B) sp2-atomic, p-hybrid
C) sp3-hybrid, p-atomic
D) sp-hybrid, p-atomic
E) σ-atomic, π-hybrid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The more effectively two atomic orbitals overlap,________.
A) the more bonding MOs will be produced by the combination
B) the higher will be the energy of the resulting bonding MO and the lower will be the energy of the resulting antibonding MO
C) the higher will be the energies of both bonding and antibonding MOs that result
D) the fewer antibonding MOs will be produced by the combination
E) the lower will be the energy of the resulting bonding MO and the higher will be the energy of the resulting antibonding MO
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The electron-domain geometry of a boron-centered compound BH3 is trigonal planar.The hybridization of the central boron atom is ________.
A) sp2
B) sp3d2
C) sp3
D) sp3d
E) sp
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to valence bond theory,which orbitals overlap in the formation of the bond in HCl?
A) 1s on H and 2p on Cl
B) 2s on H and 3p on Cl
C) 1s on H and 3s on Cl
D) 1s on H and 3p on Cl
E) 1s on H and 4p on Cl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
There is/are ________ π bond(s) in the molecule below. 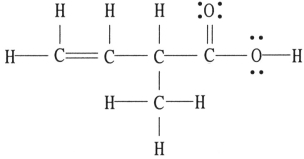
A) 0
B) 1
C) 2
D) 4
E) 16
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the molecules has a see-saw shape?
A) (i)
B) (ii)
C) (iii)
D) (iv)
E) (v)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molecular geometry of the PF4+ ion is ________.
A) octahedral
B) tetrahedral
C) trigonal pyramidal
D) trigonal planar
E) trigonal bipyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For molecules with only one central atom,how many lone pairs on the central atom guarantees molecular polarity?
A) 1
B) 2
C) 1 or 2
D) 3
E) 1 or 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The F-B-F bond angle in the BF2- ion is approximately ________.
A) 90°
B) 109.5°
C) 120°
D) 180°
E) 60°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The H-C-H bond angle in the CH4 ion is approximately ________.
A) 180
B) 120
C) 109.5
D) 60
E) 90
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to valence bond theory,which orbitals overlap in the formation of the bond in 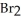 ?
?
A) 3s
B) 3p
C) 4s
D) 4p
E) 3d
Correct Answer

verified
Correct Answer
verified
True/False
Boron trifluoride has three bonding domains,and its electron domain geometry is trigonal planar.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The molecular geometry of the PHCl2 molecule is ________.
A) bent
B) trigonal planar
C) trigonal pyramidal
D) tetrahedral
E) T-shaped
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to molecular orbital theory,how many unpaired electrons are in a peroxide ion,O22-?
A) 0
B) 1/2
C) 1
D) 2
E) 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the VSEPR model,the molecular geometry of the central atom in KrF2 is ________.
A) linear
B) trigonal planar
C) tetrahedral
D) bent
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of the I atom in a IF5 molecule?
A) sp2d2
B) sp3d
C) sp3
D) sp3d2
E) sp2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The hybridization of carbon in the H-C  C-H molecule is ________.
C-H molecule is ________.
A) sp2
B) sp
C) s2p
D) s3p
E) sp3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Lewis structure of carbon monoxide is given below.The hybridizations of the carbon and oxygen atoms in carbon monoxide are ________ and ________,respectively. - :C ≡ O: +
A) sp, sp3
B) sp2, sp3
C) sp3, sp2
D) sp, sp
E) sp2, sp2
Correct Answer

verified
Correct Answer
verified
Showing 121 - 140 of 183
Related Exams