A) Viscosity
B) Surface tension
C) Volatility
D) Meniscus
E) Capillary action
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is most likely to exhibit liquid-crystalline behavior?
A) CH3CH2-C(CH3) 2-CH2CH3
B) CH3CH2CH2CH2CH2CH2CH2CH3
C) CH3CH2CH2CH2CH2- ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
On the phase diagram shown above,the coordinates of point ________ correspond to the triple point.
A) A
B) B
C) C
D) D
E) E
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Some things take longer to cook at high altitudes than at low altitudes because ________.
A) water boils at a lower temperature at high altitude than at low altitude
B) water boils at a higher temperature at high altitude than at low altitude
C) heat isn't conducted as well in low density air
D) natural gas flames don't burn as hot at high altitudes
E) there is a higher moisture content in the air at high altitude
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type(s) of intermolecular forces exist between NH3 and PO43-?
A) dispersion forces
B) dispersion forces, ion-dipole, and dipole-dipole
C) dispersion forces and dipole-dipole
D) dispersion forces and ion-dipole
E) dispersion forces, ion-dipole, dipole-dipole, and hydrogen bonds
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
A gas is ________ and assumes ________ of its container,whereas a liquid is ________ and assumes ________ of its container.
A) compressible, the volume and shape, not compressible, the shape of a portion
B) compressible, the shape, not compressible, the volume and shape
C) compressible, the volume and shape, compressible, the volume
D) condensed, the volume and shape, condensed, the volume and shape
E) condensed, the shape, compressible, the volume and shape
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which statement is true about liquids but not true about solids?
A) They flow and are highly ordered.
B) They are highly ordered and not compressible.
C) They flow and are compressible.
D) They assume both the volume and the shape of their containers.
E) They flow and are not compressible.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on the following information,which compound has the strongest intermolecular forces? Substance ΔHvap (kJ/mol) Argon (Ar) 6.3 Benzene (C6H6) 31.0 Ethanol (C2H5OH) 39.3 Water (H2O) 40.8 Methane (CH4) 9.2
A) Argon
B) Benzene
C) Ethanol
D) Water
E) Methane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The enthalpy change for converting 10.0 g of ice at -50.0 °C to water at 50.0 °C is ________ kJ.The specific heats of ice,water,and steam are 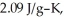
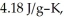 and
and 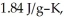 respectively.For
respectively.For 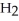 O,Δ
O,Δ 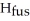 = 6.01 kJ/mol,and
= 6.01 kJ/mol,and 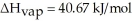 .
.
A) 12.28
B) 4.38
C) 3138
D) 6.47
E) 9.15
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The heating curve shown was generated by measuring the heat flow and temperature of a solid as it was heated.The heat flow into the sample in the segment D-E will yield the value of the ________ of this substance.
A) ΔHsub
B) ΔHvap
C) ΔHmelting
D) ΔHfusion
E) ΔHrxn
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the predominant intermolecular force in HCN?
A) dipole-dipole attraction
B) ion-dipole attraction
C) ionic bonding
D) hydrogen bonding
E) London dispersion forces
Correct Answer

verified
Correct Answer
verified
True/False
The principal source of the difference in the normal boiling points of ICl (97 °C; molecular mass 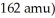 and Br2 (59 °C; molecular mass 160 amu)is both dipole-dipole interactions and London dispersion forces.
and Br2 (59 °C; molecular mass 160 amu)is both dipole-dipole interactions and London dispersion forces.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
With what compound will NH3 experience only ion-dipole intermolecular forces?
A) LiCl
B) SiH4
C) CH3I
D) C3H7OH
E) OCl2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The ________ (is) are associated with the heat energy being used up to increase distances between molecules.
A) phase change B → C
B) phase changes B → C and D → E
C) phase change D → E
D) phase change B → E
E) phase change C → E
Correct Answer

verified
Correct Answer
verified
Multiple Choice
________ liquid crystals are colored and change color with temperature changes.
A) Smectic A
B) Nematic
C) Cholesteric
D) Smectic B
E) Smectic C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has London Forces as its only intermolecular force?
A) H2O
B) CH3CH2NH2
C) HOCH2CH2OH
D) CH3CH3
E) None, all of the above exhibit dispersion forces.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound has the strongest intermolecular forces?
A) CCl4
B) CI4
C) CH4
D) H2
E) O2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the phase diagram shown above,what is the normal boiling point (°C) ?
A) 10
B) -3
C) 38
D) 29
E) 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
With what compound will NH3 experience only dispersion intermolecular forces?
A) BF3
B) LiF
C) CH3I
D) CH3OH
E) HCN
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The phase diagram of a substance is given above.The region that corresponds to the solid phase is ________.
A) w
B) x
C) y
D) z
E) x and y
Correct Answer

verified
A
Correct Answer
verified
Showing 1 - 20 of 124
Related Exams