A) HA (aq) + H2O (l) ![]() H2A+ (aq) + OH-(aq)
H2A+ (aq) + OH-(aq)
B) A- (aq) + H3O+ (aq) ![]() HA (aq) + H2O (l)
HA (aq) + H2O (l)
C) HA (aq) + OH- (aq) ![]() H2O (l) + H+ (aq)
H2O (l) + H+ (aq)
D) A- (aq) + H2O (l) ![]() HA (aq) + OH- (aq)
HA (aq) + OH- (aq)
E) A- (aq) + OH- (aq) ![]() HOA2- (aq)
HOA2- (aq)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The pH of a 0.55 M aqueous solution of hypobromous acid,HBrO,at 25.0 °C is 4.48.What is the value of Ka for HBrO?
A) 2.0 × 10-9
B) 1.1 × 10-9
C) 6.0 × 10-5
D) 3.3 × 10-5
E) 3.0 × 104
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the concentration (in M) of hydroxide ions in a solution at 25.0 °C with pH = 4.282?
A) 4.28
B) 9.72
C) 1.92 × ![]()
D) 5.22 × ![]()
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the conjugate acid of OH-?
A) O2
B) H2O
C) O-
D) O2-
E) H3O+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of an aqueous solution at 25.0 °C in which [O ![What is the pH of an aqueous solution at 25.0 °C in which [O ] is 0.0030 M? A) 5.81 B) -11.48 C) 2.52 D) -2.52 E) 11.48](https://d2lvgg3v3hfg70.cloudfront.net/TB1194/11ea7e7c_615d_c135_9a0a_812f6654bc0a_TB1194_11.jpg) ] is 0.0030 M?
] is 0.0030 M?
A) 5.81
B) -11.48
C) 2.52
D) -2.52
E) 11.48
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following aqueous solutions does the weak acid exhibit the highest percentage ionization?
A) 0.01 M H2SO3 ( ![]() = 1.4 ×
= 1.4 × ![]() )
)
B) 0.01 M HCN ( ![]() = 6.2 ×
= 6.2 × ![]() )
)
C) 0.01 M H2CO3 ( ![]() = 4.5 ×
= 4.5 × ![]() )
)
D) 0.01 M HC3H5O2 ( ![]() = 1.3 ×
= 1.3 × ![]() )
)
E) 0.01 M HOCl ( ![]() = 3.5 ×
= 3.5 × ![]() )
)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An aqueous solution of a particular compound has pH = 7.46.The compound is ________.
A) a weak base
B) a weak acid
C) a strong acid
D) a strong base
E) a salt
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The magnitude of Kw indicates that ________.
A) water autoionizes very slowly
B) water autoionizes very quickly
C) water autoionizes only to a very small extent
D) the autoionization of water is exothermic
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
The molar concentration of hydroxide ion in pure water at 25 °C is ________.
A) 1.00
B) 0.00
C) 1.0 × 10-14
D) 1.0 × 10-7
E) 7.00
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An aqueous solution contains 0.500 M NaOH at 25.0 °C.The pH of the solution is ________.
A) 0.500
B) 13.70
C) 0.301
D) 7.00
E) 13.50
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the concentration (in M) of hydroxide ions in a solution at 25.0 °C with a pOH of 3.58.
A) 2.63 × 1010
B) 3.80 × 10-11
C) 1.00 × 10-7
D) 3.80 × 103
E) 2.63 × 10-4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The base-dissociation constant,Kb,for an unknown base is 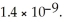 The acid-dissociation constant,Ka,for the conjugate ion is ________.
The acid-dissociation constant,Ka,for the conjugate ion is ________.
A) 1.0 × 10-7
B) 7.1 × 10-6
C) 1.4 × 10-23
D) 1.4 × 10-5
E) 7.1 × 10-4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Ka of some acid,HA,at 25.0 °C is 4.9 × 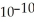 .What is the pH of a 0.050 M aqueous solution of A-?
.What is the pH of a 0.050 M aqueous solution of A-?
A) 1.0 × 10-3
B) 3.00
C) 11.00
D) 9.9 × 10-12
E) 2.5 × 10-11
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pOH of 0.606 M anilinium hydrochloride ( 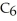
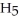 N
N 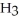 Cl) solution in water,given that
Cl) solution in water,given that 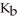 for aniline is 3.83 ×
for aniline is 3.83 × 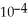 ?
?
A) 12.42
B) 1.82
C) 8.60
D) 5.40
E) 12.18
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The 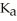 for formic acid (HCO2H) is 1.8 ×
for formic acid (HCO2H) is 1.8 × 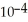 .What is the pH of a 0.20 M aqueous solution of sodium formate (NaHC
.What is the pH of a 0.20 M aqueous solution of sodium formate (NaHC 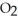 ) ?
) ?
A) 11.64
B) 5.48
C) 3.39
D) 8.52
E) 4.26
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of 0.626 M anilinium hydrochloride ( 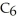
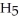 N
N 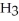 Cl) solution in water,given that
Cl) solution in water,given that 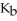 for aniline is 3.83 ×
for aniline is 3.83 × 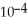 ?
?
A) 1.81
B) 5.39
C) 12.19
D) 12.42
E) 8.61
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
The conjugate base of HSO4- is ________.
A) H2SO4
B) HSO4+
C) H+
D) SO42-
E) HSO3+
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Using the data in the table,which of the conjugate acids below is the strongest acid? 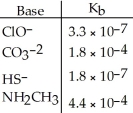
A) HClO
B) HCO3-
C) H2S
D) NH3CH3+
E) H2S and HClO
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the data in the table,which of the conjugate acids below is the weakest acid? 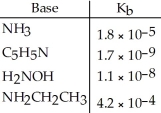
A) NH4+
B) C5H5NH+
C) NH3CH2CH3+
D) H3NOH+
E) NH4+ and NH3CH3+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following is a Br∅nsted-Lowry acid?
A) (CH3) 3NH+
B) CH3COOH
C) HF
D) HNO2
E) all of the above
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 139
Related Exams