A) atomic number.
B) mass number.
C) atomic mass.
D) molecular mass.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The number of electrons in an atom of phosphorous-31 is
A) 9
B) 15
C) 16
D) 22
Correct Answer

verified
Correct Answer
verified
Essay
Compare and contrast the mass,electrical charge,and location of the three major subatomic particles.
Correct Answer

verified
The proton has a mass of one amu,a charg...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
A substance has 13 protons,14 neutrons,and 13 electrons.Which isotope is it?
A) Al-13
B) Al-14
C) Al-27
D) Al-40
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The number of protons and neutrons in an atom are added to find the atom's
A) atomic number.
B) ionic charge.
C) number of electrons.
D) mass number.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which contains the largest number of neutrons?
A) C-12
B) P-31
C) Br-80
D) Sr-90
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In isotopic notation mass number is represented by the superscript
A) A
B) N
C) P
D) Z
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the atomic number of sodium?
A) 11
B) 16
C) 23
D) 32
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Models of the atom were proposed by Rutherford,Dalton,and Thomson.Which choice places them in the correct chronological order of their appearance?
A) Rutherford,Dalton,Thomson
B) Thomson,Rutherford,Dalton
C) Dalton,Rutherford,Thomson
D) Dalton,Thomson,Rutherford
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ions can be formed from atoms by losing or gaining electrons.Select the alternative that states the correct number of protons,neutrons,and electrons in 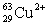 .
.
A) 29 protons,34 neutrons,31 electrons
B) 29 protons,34 neutrons,27 electrons
C) 27 protons,29 neutrons,29 electrons
D) 29 protons,63 neutrons,29 electrons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ions can be formed from atoms by losing or gaining electrons.Select the alternative that states the correct number of protons,neutrons,and electrons in 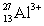 .
.
A) 13 protons,14 neutrons,and 16 electrons
B) 13 protons,14 neutrons,and 10 electrons
C) 10 protons,17 neutrons,13 electrons
D) 13 protons,27 neutrons,10 electrons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What charge does an anion possess?
A) Positive
B) Negative
C) Neutral
Correct Answer

verified
Correct Answer
verified
Essay
Define in simple terms the concept of natural abundance for the isotopes of any element and how it determines the average atomic masses of the elements.
Correct Answer

verified
Imagine taking a random sample of 100 at...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which isotope contains the same number of protons,neutrons,and electrons?
A) H-1
B) Na-23
C) C-12
D) Al-27
Correct Answer

verified
Correct Answer
verified
True/False
Dalton's atomic model states that all atoms are composed of protons,neutrons,and electrons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which isotope has the same number of protons,neutrons,and electrons?
A) Cl-35
B) Cl-37
C) P-31
D) S-32
Correct Answer

verified
Correct Answer
verified
Essay
Explain how Rutheford's gold foil experiment changed the model of the atom proposed by Thomson.
Correct Answer

verified
Thomson's model of the atom (or "plum-pu...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Sulfur occurs naturally as four isotopes: 32S with a mass of 31.9721 amu,33S with a mass of 32.9715 amu,34S 33.9679 amu,and 36S with a mass of 35.9671 amu.Based on the information that is given on the periodic table,which isotope is the most abundant?
A) (32S)
B) (33S)
C) (34S)
D) (36S)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many protons are in a neutral atom of Ar-40?
A) 18
B) 22
C) 40
D) 58
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which contains the fewest neutrons?
A) H-2
B) H-3
C) He-4
D) He-5
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 112
Related Exams