A) (-1)
B) +1
C) +2
D) +3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a double replacement reaction?
A) CuO + H2 Cu + H2O
B) HBr + KOH H2O + KBr
C) SO2 + H2O H2SO3
D) 2HI I2 + H2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following does an element have an oxidation number of +4?
A) TiO2
B) HBr
C) SO3
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following reactions is correctly balanced?
A) N2 + H 2NH3
B) 2H2O + C CO + 2H2
C) Zn + 2HCl H2 + ZnCl2
D) CO + O2 CO2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following conversion factors would allow one to calculate the amount of PbI2 produced from a given amount of NaI in the following unbalanced reaction? Pb(NO3) 2 + Nal Pbl2 + NaNO3
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction below would be considered _____ . CaCO3 + heat CaO + CO2
A) an endothermic combination.
B) an exothermic combination.
C) an endothermic decomposition.
D) an exothermic decomposition.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the equation below is properly balanced,what coefficient is in front of KCl? KClO3 KCl + O2
A) 1
B) 2
C) 3
D) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Oxygen can be prepared in the lab by heating potassium chlorate in the presence of a catalyst.The reaction is 2KClO3 2KCl + 3O2.How many moles of O2 could be obtained from one mole of KClO3?
A) 1.5
B) 3.0
C) 1.0
D) 2.0
Correct Answer

verified
Correct Answer
verified
True/False
Products are always on the right side of a chemical equation.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What substance is reduced in the following reaction? 4HCl + MnO2 Cl2 + 2H2O + MnCl2
A) Cl in HCl
B) Mn in MnO2
C) H in HCl
D) O in MnO2
Correct Answer

verified
Correct Answer
verified
True/False
The reaction Mg + 3N2 Mg3N2 is correctly balanced as written.
Correct Answer

verified
Correct Answer
verified
True/False
The reducing agent is the substance that is reduced in the reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following phrases can be used to describe the process of reduction?
A) to gain oxygen
B) to combine with hydrogen
C) to gain electrons
D) to decrease in oxidation number
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which species would be considered a spectator ion based on the following equation? Ba2+ + 2NO3- + 2Na+ + SO42+ BaSO4(s) + 2NO3- + 2Na+
A) Ba2+
B) SO42+
C) BaSO4
D) 2NO3-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the reaction 2CH4 + O2 2CO + 4H2,how many molecules of CO will be produced by the complete reaction of 1.60 grams of CH4?
A) 12.0 * 1023
B) 3.01 * 1022
C) 6.02 *1022
D) 2.00
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A container is filled with 4.0 g H2 and 5.0 g O2.The mixture is ignited to produce water according to the following equation.How much water is produced? 2H2 + O2 2H2O
A) 4.0 g
B) 5.0 g
C) 5.6 g
D) 9.0 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Acetylene,C2H2,burns according to the following reaction: C2H2+5O2 4CO2+2H2O.Suppose 1.20 g of C2H2 is mixed with 3.50 g of O2 in a closed,steel container,and the mixture is ignited.What substances will be found in the mixture left when the burning is complete?
A) CO2 and H2
B) O2,CO2,and H2O
C) C2H2,CO2,and H2O
D) O2,C2H2,CO2,and H2O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many grams of N2 are required to completely react with 3.03 grams of H2 for the following balanced chemical equation? N2 + 3H2 2NH3
A) 1.00
B) 6.00
C) 14.0
D) 28.0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which ions will appear in the total ionic equation for the following reaction? 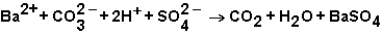
A) ![]() and
and![]()
B) ![]() and
and![]()
C) ![]() ,
,![]() ,and H+
,and H+
D) ![]() ,
,![]() ,
,![]() ,and
,and![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Haber Process is a method for the production of ammonia,an important chemical intermediate.The reaction for this process is: N2 + 3H2 2NH3.When the equation is correctly interpreted in terms of moles,how many moles of H2 will react with one mole of N2?
A) 1
B) 2
C) 3
D) 6
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 89
Related Exams