A) 255 K
B) 368 K
C) 412 K
D) 390.K
E) 466 K
Correct Answer

verified
Correct Answer
verified
Multiple Choice
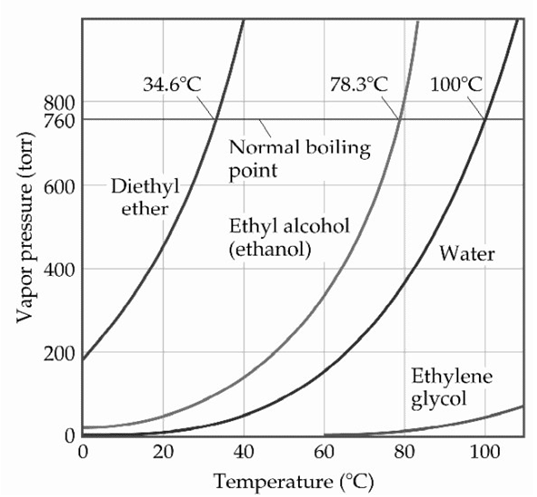 -Based on the figure above,the boiling point of diethyl ether under an external pressure of 1.32 is ________ °C.
-Based on the figure above,the boiling point of diethyl ether under an external pressure of 1.32 is ________ °C.
A) 10
B) 20
C) 30
D) 40
E) 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the edge length of a face-centred cubic unit cell made up of atoms having a radius of 128 pm?
A) 181 pm
B) 362 pm
C) 512 pm
D) 1020 pm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is TRUE?
A) Intermolecular forces are generally stronger than bonding forces.
B) The potential energy of molecules decrease as they get closer to one another.
C) Energy is given off when the attraction between two molecules is broken.
D) Increasing the pressure on a solid usually causes it to become a liquid.
E) None of the above is true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Define boiling.
A) A liquid becomes a gas.
B) A gas becomes a liquid.
C) A gas becomes a solid.
D) A solid becomes a gas.
E) A solid becomes a liquid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is expected to have the largest dispersion forces?
A) C2H4
B) C8H16
C) Cl2
D) N2H4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the type of solid for gold.
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Place the following substances in order of decreasing vapour pressure at a given temperature. BeF2 CH3OH OF2
A) CH3OH > OF2 > BeF2
B) BeF2 > OF2 > CH3OH
C) OF2 > CH3OH > BeF2
D) OF2 > BeF2 > CH3OH
E) BeF2 > CH3OH > OF2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the strongest type of intermolecular force present in CHF3?
A) ion-dipole
B) dispersion
C) hydrogen bonding
D) dipole-dipole
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the type of solid for diamond.
A) metallic atomic solid
B) ionic solid
C) nonbonding atomic solid
D) molecular solid
E) networking atomic solid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How much energy is required to vaporize 98.6 g of ethanol (C2H5OH) at its boiling point if its ΔvapH is 40.5 kJ mol-1?
A) 86.7 kJ
B) 11.5 kJ
C) 18.9 kJ
D) 52.8 kJ
E) 39.9 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the strongest type of intermolecular force present in NH2CH3?
A) dispersion
B) dipole-dipole
C) hydrogen bonding
D) ion-dipole
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the compound that exhibits hydrogen bonding as its strongest intermolecular force.
A) SCl2
B) C2H6
C) CH3OH
D) CH2F2
E) None of the above compounds exhibit hydrogen bonding.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the substance with the lowest vapour pressure at a given temperature.
A) CO2
B) BeCl2
C) BF3
D) He
E) PF5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Lithium crystallizes in a body-centred cubic structure.What is the coordination number of each atom?
A) 4
B) 6
C) 8
D) 12
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is considered an ionic solid?
A) (NH4) 2CO3
B) CCl4
C) SeBr2
D) XeF4
E) None of these is an ionic solid.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the substance with the highest viscosity in the liquid phase.
A) gasoline
B) water
C) corn syrup
D) motor oil
E) tea
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Place the following substances in order of decreasing boiling point. N2 O2 H2
A) O2 > H2 > N2
B) N2 > H2 > O2
C) N2 > O2 > H2
D) O2 > N2 > H2
E) H2 > N2 > O2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Place the following compounds in order of decreasing strength of intermolecular forces. HF O2 CO2
A) HF > CO2 > O2
B) HF > O2 > CO2
C) O2 > CO2 > HF
D) CO2 > HF > O2
E) CO2 > O2 > HF
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which substance below has the strongest intermolecular forces?
A) A2X,ΔvapH = 39.6 kJ mol-1
B) BY2,ΔvapH = 26.7 kJ mol-1
C) C3X2,ΔvapH = 36.4 kJ mol-1
D) DX2,ΔvapH = 23.3 kJ mol-1
E) EY3,ΔvapH = 21.5 kJ mol-1
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 127
Related Exams