A) 1p, K.
B) 2s, K.
C) 1s, L.
D) 2p, L.
E) 3f, M.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An electron in a hydrogen atom has principal quantum number n = 4.How many possible values of the orbital quantum number l could it have?
A) 8
B) 9
C) 3
D) 4
E) 10
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the orbital quantum number is l = 4,which one of the following is a possible value for the principal quantum number n?
A) 1
B) 2
C) 3
D) 4
E) 8
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the wavelength of the Kα line for Mercury?
A) 1.94 × 10-11 m
B) 5.38 × 10-12 m
C) 1.85 × 10-11 m
D) 3.02 × 10-12 m
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the minimum speed needed by a ground-state hydrogen atom for its kinetic energy to be enough to ionize the atom in a collision? (1 eV = 1.60 × 10-19 J, mH≈ mproton =1.67 x 10-27 kg)
A) 44.2 km/s
B) 21.7 km/s
C) 66.5 km/s
D) 113 km/s
E) 51.0 km/s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many possible sets of quantum numbers (electron states) are there in the 5f subshell?
A) 2
B) 6
C) 8
D) 10
E) 14
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the principal quantum number of an electron is n = 5,which one of the following is NOT an allowed magnetic quantum number ml for the electron?
A) 0
B) 2
C) 3
D) 4
E) 5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If two electrons in the same atom have the same four quantum numbers,then they must have the same energy.
A) True
B) False
C) They cannot both have the same four quantum numbers.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct electronic configuration for ground state carbon,which has 6 electrons?
A) 1s22s22p2
B) 1s12p1
C) 1s12s22p1
D) 1s12s12p1
E) 1s22s22p4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A muonic hydrogen atom is a proton orbited by a muon (a particle with the same charge as an electron and 207 times its mass) in which the mass of the muon is significant relative to the mass of the proton.What would be the radius of the smallest muon orbit in a muonic hydrogen atom?
A) 1.03 × 10-8 m
B) 1.10 × 10-8 m
C) 2.56 × 10-13 m
D) 2.84 × 10-13 m
E) 5.29 × 10-11 m
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The magnitude of the orbital angular momentum L of an electron in a certain atom is equal to 3.464ħ.Which one of the following numbers could be the principal quantum number n of the electron?
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An electron in a hydrogen atom is in the n = 7 shell.How many possible values of the orbital quantum number l could it have?
A) 6
B) 7
C) 15
D) 33
E) 98
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A three-dimensional potential well has potential U0 = 0 in the region 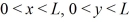 ,and
,and 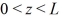 and infinite potential otherwise.The ground state energy of a particle in the well is E0.What is the energy of the first excited state,and what is the degeneracy of that state?
and infinite potential otherwise.The ground state energy of a particle in the well is E0.What is the energy of the first excited state,and what is the degeneracy of that state?
A) 2E0, single degeneracy
B) 2E0, triple degeneracy
C) 3E0, single degeneracy
D) 6E0, single degeneracy
E) 6E0, triple degeneracy
Correct Answer

verified
Correct Answer
verified
Short Answer
An atom with 5 electrons is in its ground state.How many electrons are in its outermost shell?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An electron in a hydrogen atom has orbital quantum number l = 4.How many possible values of the magnetic quantum number ml could it have?
A) 4
B) 10
C) 5
D) 9
E) 3
Correct Answer

verified
Correct Answer
verified
Short Answer
Consider the n = 9 shell. (a)What is the largest value of the orbital quantum number,l,in this shell? (b)How many electrons can be placed in this shell?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom in a state with its orbital quantum number l = 1 decays to its ground state (with l = 0) .A photon of wavelength 630.000 nm is emitted in the process.When the same process takes place in the presence of an intense magnetic field,the following change in the spectrum is observed.With the magnetic field present,one of the emitted lines observed now has a wavelength of 630.030 nm.Which of the following wavelengths would you expect to be also present?
A) 629.970 nm
B) 630.060 nm
C) 630.090 nm
D) 630.120 nm
E) 629.910 nm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the radius of the smallest electron orbit in a singly-ionized helium atom?
A) 1.32 × 10-11 m
B) 2.65 × 10-11 m
C) 5.29 × 10-11 m
D) 1.06 × 10-10 m
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Doubly-ionized lithium is a hydrogen-like ion,in which the nucleus with charge 3e is orbited by a single electron.What wavelength light would be emitted from Li++ as the electron dropped from the n = 4 to the n = 3 orbit?
A) 16.9 µm
B) 1.88 µm
C) 470 nm
D) 208 nm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For each value of the principal quantum number n,what are the possible values of the electron spin quantum number ms? (There may be more than one correct choice.)
A) 0
B) +1/2
C) -1/2
D) +3/2
E) -3/2
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 53
Related Exams