A) C
B) Zn
C) Mg
D) O
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Metalloids are located where on the periodic table?
A) left side
B) right side
C) middle
D) zig-zag diagonal line
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Group 7A elements are also called:
A) noble gases.
B) halogens.
C) alkaline earth metals.
D) alkali metals.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Group 1A elements are also called:
A) noble gases.
B) halogens.
C) alkaline earth metals.
D) alkali metals.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the statements about the discovery of electrons is FALSE?
A) Because atoms are neutral,the existence of a negatively charged particle implied there must be a positively charged component of an atom.
B) Thomson proposed that electrons were small particles held within a positively charged sphere.
C) Rutherford proved the plum-pudding model correct.
D) The negatively charged electron is located outside the nucleus.
E) All of the above statements are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A fictional element named Nivadium is found to have three naturally occurring isotopes with the natural abundances shown here: 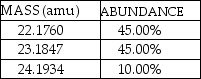 The calculated atomic mass of Nivadium is
The calculated atomic mass of Nivadium is
A) 7.61 amu
B) 22.83 amu
C) 23.18 amu
D) 69.55 amu
E) none of the above
Correct Answer

verified
Correct Answer
verified
Showing 101 - 106 of 106
Related Exams