A) 1° alkyl halides
B) 3° alkyl halides
C) vinyl halides
D) aryl halides
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What do the Suzuki reaction, the Heck reaction, and the organocuprate reaction all have in common when they react with an alkyl halide?
A) All reactions form new carbon-carbon bonds.
B) They all use palladium as a catalyst in one step of the reaction.
C) They are all stereospecific reactions.
D) They all require harsh conditions.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement below best explains what is meant by the statement, "An organocuprate reaction with a vinyl halide is stereospecific"?
A) The reaction of a specific stereoisomer with the R2CuLi reagent will yield that particular stereoisomer as the product.
B) The reaction of a vinyl halide with the R2CuLi reagent will only yield the cis product.
C) The reaction of a vinyl halide with the R2CuLi reagent will only yield the trans product.
D) The reaction of a vinyl halide with the R2CuLi reagent will only yield one enantiomer product-either R or S configuration.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the structure of the major organic product that results from the following reaction. 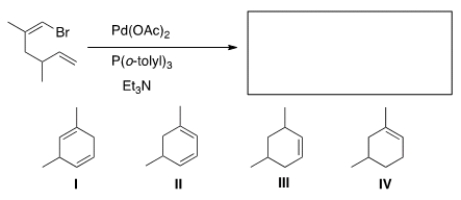
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Organocuprate reagents (R2CuLi) react with several compounds. Which listed reaction is not correct?
A) Acid chlorides react with organocuprate reagents to form ketones.
B) Epoxides react with organocuprate reagents to form alcohols.
C) Alkyl halides react with organocuprate reagents to form coupling products containing a new carbon-carbon bond.
D) Carbon dioxide reacts with organocuprate reagents to form carboxylic acids.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What would be the starting material for the following product that was made via a Grubbs catalyst? 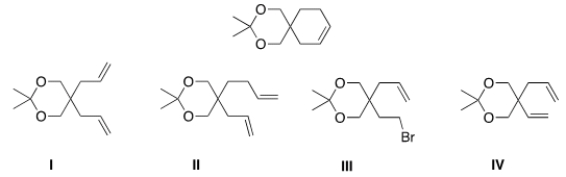
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following descriptions does not apply to methylene?
A) Methylene is sp2 hybridized.
B) Methylene is a neutral, reactive intermediate.
C) Methylene is a radical intermediate.
D) The formula of methylene is :CH2.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product of the following reaction? 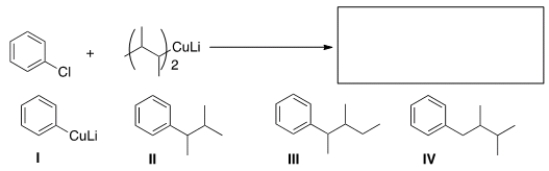
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product of the following reaction? 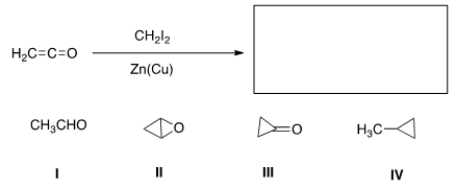
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the statement below that is not true about the Suzuki reaction.
A) The product of the Suzuki reaction is completely stereospecific.
B) The Suzuki reaction involves both an organoborane reagent and an organopalladium catalyst.
C) The Suzuki reaction forms more highly substituted alkenes.
D) The Suzuki reaction involves an oxidative addition followed by a reductive elimination.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is not true about a carbene?
A) A carbene is a neutral reactive intermediate.
B) A carbene contains a divalent carbon.
C) A carbene is sp3 hybridized.
D) A carbene is surrounded by six electrons.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
As shown below, when cis-2-butene reacts with dichlorocarbene, only the cis-1,1-dichloro-2,3-dimethylcyclopropane is formed. What can we conclude about the nature of the reaction mechanism? 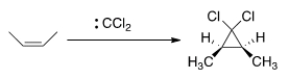
A) The mechanism is an SN1 mechanism.
B) The mechanism is a concerted.
C) The mechanism proceeds through a radical intermediate.
D) The mechanism is an E2 mechanism.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the structure of the organic product Y formed in the following reaction sequence. 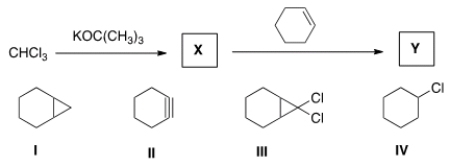
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the major organic product of the following reaction. 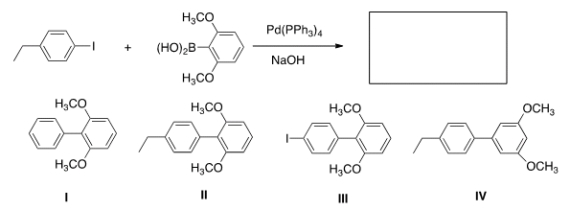
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Coupling reactions with vinyl halides are stereospecific. Which choice below best describes the expected product when trans-1-bromo-1-hexene reacts with (CH3) 2CuLi?
A) The reaction will only yield a trans-alkene.
B) The reaction will only yield a cis-alkene.
C) The reaction will only yield one enantiomeric product with R configuration.
D) The reaction will only yield one enantiomeric product with S configuration.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major organic product of the following reaction? 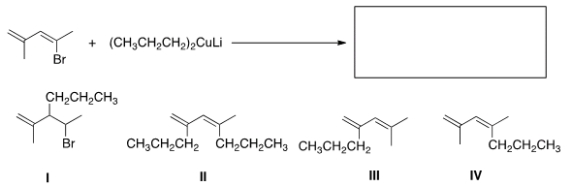
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is true about the use of the Grubbs catalyst?
A) The Grubbs catalyst is used in carbon-carbon coupling reactions.
B) The Grubbs catalyst is used in alkene metathesis.
C) The Grubbs catalyst is used in carbene formation.
D) The Grubbs catalyst is used with palladium as a co-catalyst.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What starting material could yield the following compound if Simmons-Smith conditions were used? 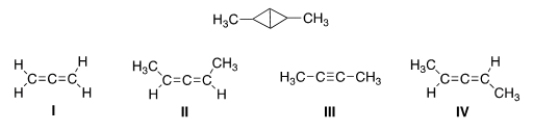
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the major organic product of the following reaction. 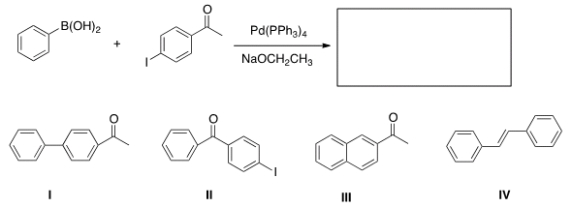
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What products are formed when diazomethane is heated?
A) methane gas and nitrogen gas
B) propene gas and nitrogen gas
C) methyl radical and nitrogen gas
D) methylene and nitrogen gas
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 41
Related Exams