A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which diene would be least reactive toward Diels-Alder addition of maleic anhydride? 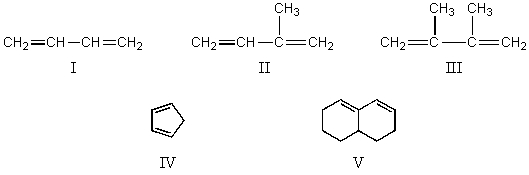
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these dienes can undergo the Diels-Alder reaction?
A) 1,3-Pentadiene
B) 1,4-Pentadiene
C) 1,2-Butadiene
D) 1,4-Cyclohexadiene
E) All of the above can undergo the Diels-Alder reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which would be the best synthesis? 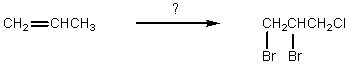
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Short Answer
Conjugated dienes routinely undergo 1,2 and 1,4 addition reactions with a variety of electrophilic reagents;this suggests that ___________ are likely intermediates during these reactions.
Correct Answer

verified
allylic ca...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Select the structure of the conjugated diene. 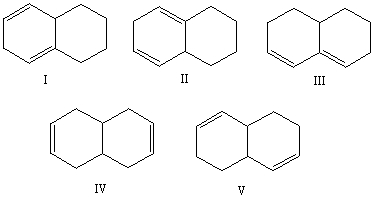
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would afford a synthesis of the following compound? 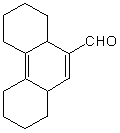
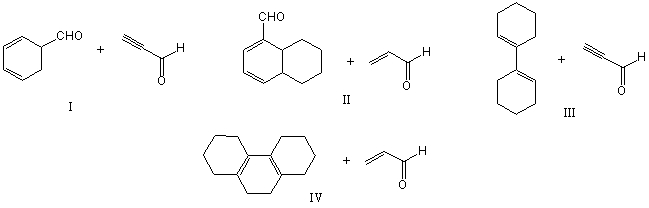
A) I
B) II
C) III
D) IV
E) None of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following dienes would you expect to be the most stable?
A) CH3CH2CH=CHCH2CH=CHCH3
B) CH3CH=CHCH=CHCH2CH3
C) CH2=CHCH2CH2CH2CH=CH2
D) CH2=CHCH=CHCH2CH2CH3
E) CH3CH=C(CH3) CH=CHCH2CH3
Correct Answer

verified
Correct Answer
verified
Short Answer
There are three types of polyenes (molecules containing two or more double bonds).They are: _________________.
Correct Answer

verified
conjugated...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Which diene and dienophile would you use to prepare the following molecule using a Diels-
Alder cycloaddition reaction: 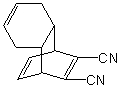
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these conjugated dienes can undergo a Diels-Alder reaction? 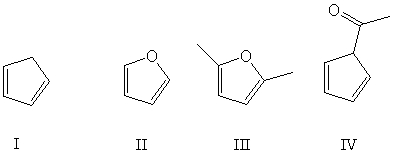
A) I
B) II
C) III
D) IV
E) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
From the standpoint of reactivity,which is the poorest choice of dienophile to react with 2,3-dimethyl-1,3-butadiene in a Diels-Alder reaction? 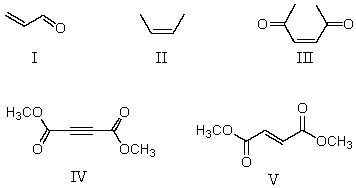
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following dieneophiles is most reactive in a Diels-Alder reaction: 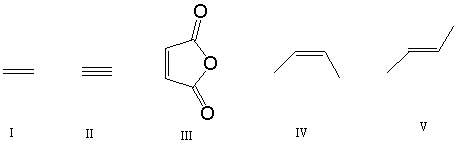
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which alkene would you expect to be most stable?
A) CH2=CHCH2CH2CH=CH2
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which diene and dienophile would you choose to synthesize the following compound? 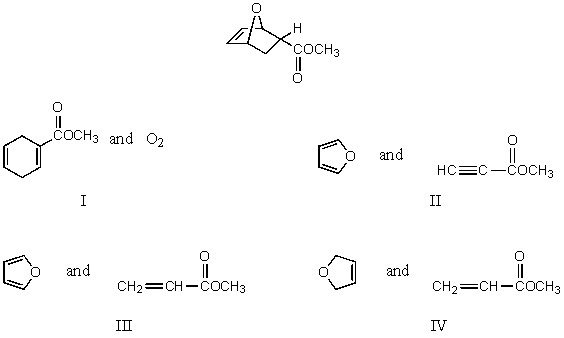
A) I
B) II
C) III
D) IV
E) None of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The accompanying diagram implies that: 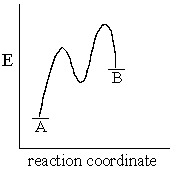
A) the formation of B from A would be favored at high temperature.
B) the more stable product forms more rapidly from the intermediate species.
C) the formation of B from the intermediate is the rate-limiting step in the transformation of A into B
D) the formation of B from A is not a concerted reaction.
E) All of the above statements are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An unsaturated product results from the reaction of cyclohexene with which of these?
A) Br2/CCl4 at 25 C
B) NBS/CCl4,ROOR
C) HCl,ROOR
D) HCl,no peroxides
E) More than one of these
Correct Answer

verified
Correct Answer
verified
Essay
Complete the following sequence of reactions,giving structural details of all key intermediates. 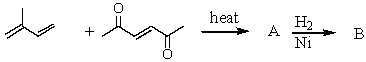
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is an untrue statement concerning the Diels-Alder reaction?
A) The reaction is a syn addition.
B) The diene must be in the s-cis conformation to react.
C) Most Diels-Alder reactions are reversible.
D) Generally,the adduct formed most rapidly is the exo product.
E) Electron donating groups on the diene and electron withdrawing groups on the dieneophile favor adduct formation.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the major product of the following reaction? 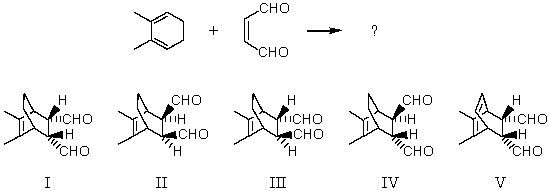
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 166
Related Exams