A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Essay
Suggest a reasonable synthetic strategy to carry out the following transformation. 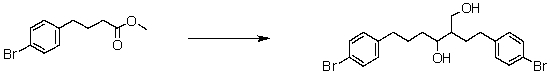
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What product(s) is (are) likely to be obtained upon Dieckmann condensation of the following substance? 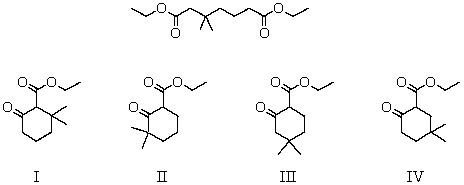
A) I and II
B) II and III
C) III and IV
D) I and III
E) II and IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What would be the major product,B,of the following reaction sequence? 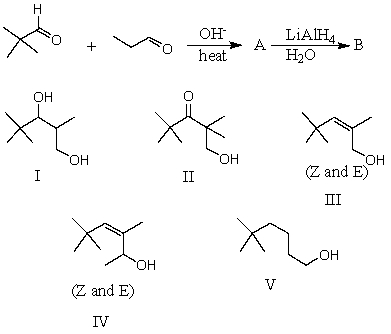
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Cyclization reactions,such as the Dieckmann condensation,are best carried out using fairly dilute solutions of the compound to be cyclized.Why is this so?
A) It then is possible to use less base.
B) The reagents generally are expensive.
C) A smaller amount of the compound to be cyclized can be used.
D) Intermolecular condensation is minimized at low concentration.
E) The concentration factor is unimportant.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound could be prepared via Dieckmann condensation? 
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Essay
What is the final product of the following reaction sequence? Give structural
details of all significant intermediates. 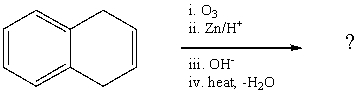
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Robinson annulation reaction which produces 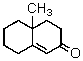 uses which of the following as starting materials?
uses which of the following as starting materials? 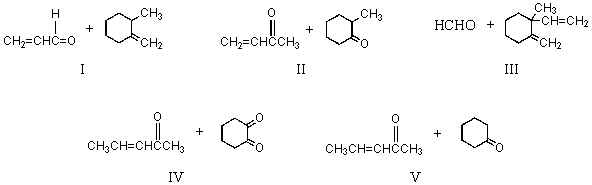
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
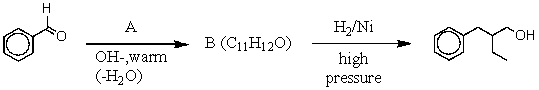 -What is the intermediate B in the synthesis shown above?
-What is the intermediate B in the synthesis shown above? 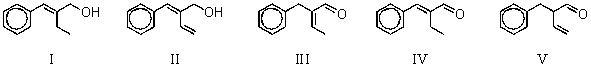
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these is not a reversible process?
A) Base-promoted ester hydrolysis
B) Acid-catalyzed ester hydrolysis
C) Aldol addition
D) Claisen condensation
E) Acetal formation
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What starting compound(s) would you use in an aldol reaction to prepare as the major product: 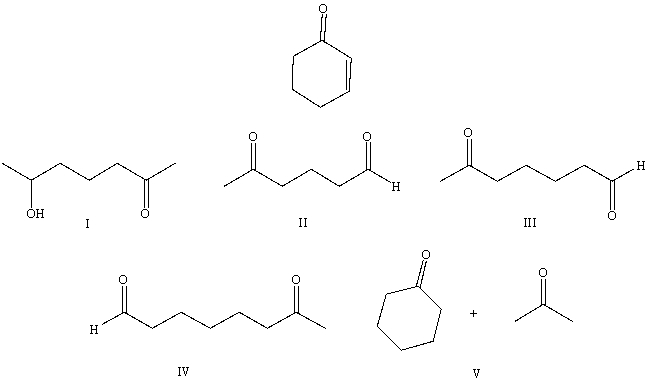
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Showing 121 - 131 of 131
Related Exams