A) CH3CH2OD
B) CH3CH2CH2CH3
C) CH2=CH2
D) CH3CH2D
E) CH3CH2OCH2CH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these is not a Lewis acid?
A) AlCl3
B) H3O+
C) FeCl3
D) SO3
E) C4H10
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a conjugate acid - conjugate base pair (in that order) ?
A) H3PO4,H2PO4-
B) HBF4,BF4-
C) CH3CH2OH,CH3CH2O--
D) H3O+,H2O
E) HPO42-,H2PO4-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 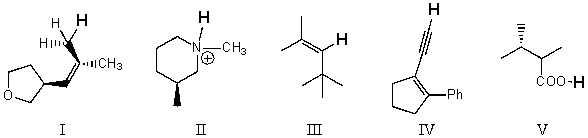
A) I > II > III > IV > V
B) V > IV > II > III > I
C) V > II > IV > III > I
D) II > V > IV > III > I
E) V > IV > III > II > I
Correct Answer

verified
Correct Answer
verified
True/False
For a reaction that has positive change in entropy,increasing the temperature of the reaction will help make the reaction energetically favorable to form products.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which acid-base reaction would not take place as written?
A) CH3Li + CH3CH2CH2CH2NH2 CH4 + CH3CH2CH2CH2NHLi
B) CH3C CH + NaOCH3 CH3C CNa + CH3OH
C) HC CNa + H2O HC CH + NaOH
D) CH3OH + NaNH2 CH3ONa + NH3
E) CH3CO2H + CH3ONa CH3CO2Na + CH3OH
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The compound aniline,C6H5NH2,has weakly basic properties in aqueous solution.In this other solvent,aniline would behave as a strong base.
A) CH3OH
B) CH3CH2OH
C) CF3CO2H
D) Liquid NH3
E) CH3(CH2) 4CH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the bold-faced hydrogens for the following compounds from most acidic to least acidic. 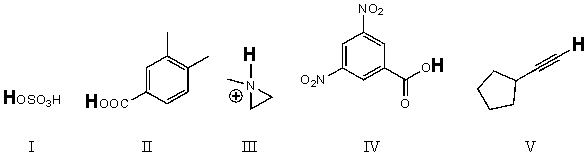
A) IV > II > III > V > I
B) II > IV > I > V > III
C) IV > I > III > II > V
D) I > IV > II > V > III
E) I > IV > II > III > V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Adding methyllithium,CH3Li,to ethanol produces:
A) CH3CH2Li + CH3OH
B) CH3CH2OLi + CH4
C) CH3CH2OCH3 + LiH
D) All of these choices.
E) No reaction takes place.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which reaction will yield CH3CH2-D?
A) CH3CH3 + D2O
B) CH3CH2Li + D2O
C) CH3CH2OLi + D2O
D) CH3CH2OH + D2O
E) More than one of these choices.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the reaction conditions could afford the following transformation? 
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) All of these choices would work.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the following reaction which chemical species is acting like a nucleophile? 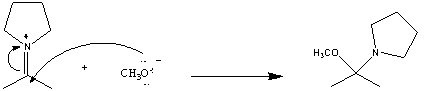
A) ![]()
B) ![]()
C) CH3O-
D) two of these choices
E) there is no nucleophile in this reaction
Correct Answer

verified
Correct Answer
verified
Essay
Why do water-insoluble carboxylic acids dissolve in aqueous sodium hydroxide?
Correct Answer

verified
Because they are con...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Based on the position of the central atom in the periodic chart,we predict that the strongest acid of the following is:
A) H2O
B) H2S
C) H2Se
D) H2Te
E) All acids are equal in strength.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these is not a true statement?
A) All Lewis bases are also Bronsted-Lowry bases.
B) All Lewis acids contain hydrogen.
C) All Bronsted-Lowry acids contain hydrogen.
D) All Lewis acids are electron deficient.
E) According to the Bronsted-Lowry theory,water is both an acid and a base.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following substances has a hydrogen atom with pKa ≈ 25?
A) CH3CH2CH2CH2CO2H
B) CH3CHCH2C≡CCH3
C) CH3CH2CH2C≡CH
D) CH3CH2CH2CH2CH=CH2
E) CH3CH2CH2CH2NH2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An acid,HA,has the following thermodynamic values for its dissociation in water at 27 ºC: H = -8.0 kJ mol-1; S = -70 J K-1mol-1.The G for the process is:
A) +29 kJ mol-1
B) +13 kJ mol-1
C) -6.1 kJ mol-1
D) -13 kJ mol-1
E) -29 kJ mol-1
Correct Answer

verified
Correct Answer
verified
Essay
Briefly,but clearly,explain why the -OH hydrogen in acetic acid (CH3CO2H)is more acidic than in ethanol (C2H5OH).
Correct Answer

verified
The greater acidity of the -OH hydrogen ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What deuterium reactant would give the following product? 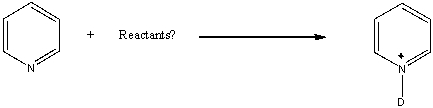
A) D2O
B) D3O+
C) NaOD
D) CH3OD
E) None of these choices.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A group of acids arranged in order of decreasing acidity is: HNO3 > CH3COOH > C6H5OH > H2O > HC CH What is the arrangement of the conjugate bases of these compounds in decreasing order of basicity?
A) NO3- > CH3COO- > C6H5O- > OH- > HC C-
B) CH3COO- > C6H5O- > NO3- > OH- > HC C-
C) C6H5O- > NO3- > HC C- > OH- > CH3COO-
D) HC C- > OH- > C6H5O- > CH3COO- > NO3-
E) No prediction of relative base strength is possible.
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 149
Related Exams