Correct Answer

verified
When we draw the Lewis dot structure for...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Draw a molecular orbital diagram for O2 and N2.Using molecular orbital theory,explain why the removal of one electron in O2 strengthens bonding,while the removal of one electron in N2 weakens bonding.
Correct Answer

verified
 The bond order for N2 is 3,b...
The bond order for N2 is 3,b...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
When a carbon atom has sp3 hybridization,it has
A) four bonds
B) three bonds and one bond
C) two bonds and two bonds
D) one bond and three bonds
E) four bonds
Correct Answer

verified
Correct Answer
verified
True/False
The hybridization of a molecule is measured to determine the shape of the molecule.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the skeletal structure shown below: N-C-C-N Draw the Lewis structure and answer the following: -How many pi bonds does the molecule contain?
A) 0
B) 2
C) 4
D) 6
E) 7
Correct Answer

verified
Correct Answer
verified
Short Answer
__________ is the difference between the number of bonding electrons and the number of antibonding electrons divided by two.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following has the largest bond order?
A) N2
B) N2-
C) N22-
D) N2+
E) N22+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A species has the following MO configuration: ( 1s) 2( 1s*) 2( 2s) 2( 2s*) 2( 2p) 2( 2p) 2 This substance is
A) paramagnetic with one unpaired electron
B) paramagnetic with two unpaired electrons
C) paramagnetic with three unpaired electrons
D) paramagnetic with four unpaired electrons
E) diamagnetic
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the molecule and the following hybridization choices: 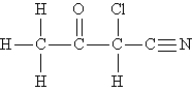 -What is the hybridization of the nitrogen atom?
-What is the hybridization of the nitrogen atom?
A) sp
B) sp2
C) sp3
D) dsp3
E) d2sp3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about the species CN- is false?
A) It is paramagnetic.
B) The total number of electrons is 14.
C) Its bond order is 3.
D) It has two pi bonds.
E) All of these are true.
Correct Answer

verified
Correct Answer
verified
True/False
According to MO theory,F2 should be diamagnetic.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the nitrogen-containing molecules below is paramagnetic in its lowest energy state?
A) N2
B) NO
C) NH3
D) N2H4
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Tetracyanoethylene has the skeleton shown below: 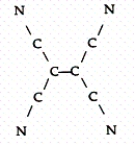 From its Lewis structure determine the following:
-How many of the atoms are sp hybridized?
From its Lewis structure determine the following:
-How many of the atoms are sp hybridized?
A) 2
B) 4
C) 6
D) 8
E) 10
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The hybridization of the central atom in I3- is:
A) sp
B) sp2
C) sp3
D) dsp3
E) d2sp3
Correct Answer

verified
Correct Answer
verified
True/False
When an electron pair is shared in the area centered on a line joining the atoms,a (sigma)bond is formed.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For which of the following diatomic molecules would the bond order become greater if an electron is removed (i.e. ,if the molecule is converted to the positive ion in its ground state) ?
A) B2
B) C2
C) P2
D) F2
E) Na2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
As the bond order of a bond increases,the bond energy ______ and the bond length ______.
A) increases,increases
B) decreases,decreases
C) increases,decreases
D) decreases,increases
E) More information is needed to answer this question.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The hybridization of the central atom in NO3- is
A) p3
B) sp2
C) sp3
D) sp
E) dsp2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a molecule demonstrates paramagnetism,then : I.The substance can have both paired and unpaired electrons. II.The bond order is not a whole number. III.It can be determined by drawing a Lewis structure. IV.It must be an ion.
A) I,II
B) I,II,IV
C) II,III
D) I only
E) All of the above are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For how many of the following does the bond order decrease if you add one electron to the neutral molecule? B2,C2,P2,F2
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 101
Related Exams