A) 1
B) 7
C) 14
D) 15
E) 126
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a student performs an endothermic reaction in a calorimeter,how does the calculated value of H differ from the actual value if the heat exchanged with the calorimeter is not taken into account?
A) " Hcalc" would be more negative because the calorimeter always absorbs heat from the reaction.
B) " Hcalc" would be less negative because the calorimeter would absorb heat from the reaction.
C) " Hcalc' would be more positive because the reaction absorbs heat from the calorimeter.
D) " Hcalc" would be less positive because the reaction absorbs heat from the calorimeter.
E) " Hcalc" would equal the actual value because the calorimeter does not absorb heat.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Two metals of equal mass with different heat capacities are subjected to the same amount of heat.Which undergoes the smallest change in temperature?
A) The metal with the higher heat capacity.
B) The metal with the lower heat capacity.
C) Both undergo the same change in temperature.
D) You need to know the initial temperatures of the metals.
E) You need to know which metals you have.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is/are true? I.q (heat) is a state function because H is a state function and q = H. II.When 50.0 g of aluminum at 20.0°C is placed in 50.0 mL of water at 30.0°C,the H2O will undergo a smaller temperature change than the aluminum.(The density of H2O = 1.0 g/mL,specific heat capacity of H2O = 4.18 J/g°C,specific heat capacity of aluminum = 0.89 J/g°C) III.When a gas is compressed,the work is negative since the surroundings are doing work on the system and energy flows out of the system. IV.For the reaction (at constant pressure) 2N2(g) + 5O2(g) 2N2O5(g) ,the change in enthalpy is the same whether the reaction takes place in one step or in a series of steps.
A) I,II,IV
B) II,III
C) II,III,IV
D) II,IV
E) All of the above statements are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
CH4(g) + 4Cl2(g) CCl4(g) + 4HCl(g) , H = -434 kJ Based on the above reaction,what energy change occurs when 1.2 moles of methane (CH4) reacts?
A) 5.2 105 J are released.
B) 5.2 105 J are absorbed.
C) 3.6 105 J are released.
D) 3.6 105 J are absorbed.
E) 4.4 105 J are released.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following statements concerning petroleum are all true except:
A) It is a thick,dark liquid composed mostly of hydrocarbons.
B) It must be separated into fractions (by boiling) in order to be used efficiently.
C) Some of the commercial uses of petroleum fractions include gasoline and kerosene.
D) It was probably formed from the remains of ancient marine organisms.
E) All of its hydrocarbon chains contain the same number of carbon atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the lab,you mix two solutions (each originally at the same temperature) and the temperature of the resulting solution decreases.Which of the following is true?
A) The chemical reaction is releasing energy.
B) The energy released is equal to s m T.
C) The chemical reaction is absorbing energy.
D) The chemical reaction is exothermic.
E) More than one of these.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The H value for the reaction 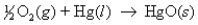 is -90.8 kJ.How much heat is released when 66.9 g Hg is reacted with oxygen?
is -90.8 kJ.How much heat is released when 66.9 g Hg is reacted with oxygen?
A) 0.333 kJ
B) 6.07 103 kJ
C) 30.3 kJ
D) 90.8 kJ
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements correctly describes the signs of q and w for the following exothermic process at P = 1 atm and T = 370 K? H2O(g) H2O(l)
A) q and w are negative.
B) q is positive,w is negative.
C) q is negative,w is positive.
D) q and w are both positive.
E) q and w are both zero.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You take 295.5 g of a solid at 30.0°C and let it melt in 425 g of water.The water temperature decreases from 85.1°C to 30.0°C.Calculate the heat of fusion of this solid.
A) 160 J/g
B) 166 J/g
C) 331 J/g
D) 721 J/g
E) cannot solve without the heat capacity of the solid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the equation S(s) + O2(g) SO2(g) , H = -296 kJ,which of the following statement(s) is (are) true? I.The reaction is exothermic. II.When 0.500 mole sulfur is reacted,148 kJ of energy is released. III.When 32.0 g of sulfur are burned,2.96 105 J of energy is released.
A) All are true.
B) None is true.
C) I and II are true.
D) I and III are true.
E) Only II is true.
Correct Answer

verified
Correct Answer
verified
Short Answer
__________ involves the transfer of energy between two objects due to a temperature difference.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 5.0 kJ of energy is added to a 15.5-g sample of water at 10.°C,the water is
A) boiling
B) completely vaporized
C) frozen solid
D) decomposed
E) still a liquid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The heat combustion of acetylene,C2H2(g) ,at 25°C is -1299 kJ/mol.At this temperature, Hf° values for CO2(g) and H2O(l) are -393 and -286 kJ/mol,respectively.Calculate Hf° for acetylene.
A) 2376 kJ/mol
B) 625 kJ/mol
C) 227 kJ/mol
D) -625 kJ/mol
E) -227 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction H2O(l) H2O(g) at 298 K and 1.0 atm, H is more positive than E by 2.5 kJ/mol.This quantity of energy can be considered to be
A) the heat flow required to maintain a constant temperature
B) the work done in pushing back the atmosphere
C) the difference in the H-O bond energy in H2O(l) compared to H2O(g)
D) the value of H itself
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 45.9 g sample of a metal is heated to 95.2°C and then placed in a calorimeter containing 120.0 g of water (c = 4.18 J/g°C) at 21.6°C.The final temperature of the water is 24.5°C.Which metal was used?
A) Aluminum (c = 0.89 J/g°C)
B) Iron (c = 0.45 J/g°C)
C) Copper (c = 0.20 J/g°C)
D) Lead (c = 0.14 J/g°C)
E) none of these
Correct Answer

verified
Correct Answer
verified
True/False
When a system performs work on the surroundings,the work is reported with a negative sign.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
On a cold winter day,a steel metal fence post feels colder than a wooden fence post of identical size because:
A) The specific heat capacity of steel is higher than the specific heat capacity of wood.
B) The specific heat capacity of steel is lower than the specific heat capacity of wood.
C) Steel has the ability to resist a temperature change better than wood.
D) The mass of steel is less than wood so it loses heat faster.
E) Two of the above statements are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A fuel-air mixture is placed in a cylinder fitted with a piston.The original volume is 0.310-L.When the mixture is ignited,gases are produced and 935 J of energy is released.To what volume will the gases expand against a constant pressure of 635 mmHg,if all the energy released is converted to work to push the piston?
A) 10.7 L
B) 8.02 L
C) 11.4 L
D) 11.0 L
E) 1.78 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The heat of formation of Fe2O3(s) is -826.0 kJ/mol.Calculate the heat of the reaction 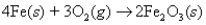 when a 53.99-g sample of iron is reacted.
when a 53.99-g sample of iron is reacted.
A) -199.6 kJ
B) -399.2 kJ
C) -798.5 kJ
D) -1597 kJ
E) -2.230 104 kJ
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 86
Related Exams