A) the possibility of chemical reaction between molecules
B) the finite volume of molecules
C) the quantum behavior of molecules
D) the fact that average kinetic energy is inversely proportional to temperature
E) the possibility of phase changes when the temperature is decreased or the pressure is increased
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What volume of 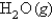 measured at STP is produced by the combustion of 6.27 g of natural gas
measured at STP is produced by the combustion of 6.27 g of natural gas 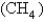 according to the following equation?
according to the following equation? 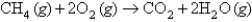
A) 8.76 L
B) 17.5 L
C) 4.38 L
D) 19.1 L
E) 3.14 L
Correct Answer

verified
Correct Answer
verified
True/False
The diffusion of a gas is faster than the effusion of a gas.
Correct Answer

verified
Correct Answer
verified
True/False
The pressure a gas would exert under ideal conditions is always greater than the observed pressure of a real gas.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following properties of a real gas is related to the b coefficient in the van der Waals equation?
A) Real gases consist of molecules or atoms that have volume.
B) The average speed of the molecules of a real gas increases with temperature.
C) There are attractive forces between atoms or molecules of a real gas.
D) The rate of effusion of a gas is inversely proportional to the square root of the molecular weight of the gas.
E) None of these.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is true concerning ideal gases?
A) The temperature of the gas sample is directly related to the average velocity of the gas particles.
B) At STP,1.0 L of Ar(g) contains about twice the number of atoms as 1.0 L of Ne(g) since the molar mass of Ar is about twice that of Ne.
C) A gas exerts pressure as a result of the collisions of the gas molecules with the walls of the container.
D) The gas particles in a sample exert attraction for one another.
E) All of the above are false.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Body temperature is about 309 K.On a cold day,what volume of air at 276 K must a person with a lung capacity of 2.2 L breathe in to fill the lungs?
A) 2.46 L
B) 1.97 L
C) 2.08 L
D) 3.93 L
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Standard pressure for gases is
A) 0 atm
B) 1 atm
C) 100 atm
D) dependent upon temperature
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 142-mL sample of gas is collected over water at 22°C and 753 torr.What is the volume of the dry gas at STP? (The vapor pressure of water at 22°C = 20.torr)
A) 122 mL
B) 162 mL
C) 136 mL
D) 111 mL
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Gases generally have
A) low density
B) high density
C) closely packed particles
D) no increase in volume when temperature is increased
E) no decrease in volume when pressure is increased
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What volume does 40.5 g of N2 occupy at STP?
A) 64.8 L
B) 1.81 L
C) 32.4 L
D) 50.7 L
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the ratio of the effusion rates of N2 and N2O.
A) 0.637
B) 1.57
C) 1.25
D) 0.798
E) 1.61
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Zinc metal is added to hydrochloric acid to generate hydrogen gas,which is collected over a liquid whose vapor pressure is the same as pure water at 20.0°C (18 torr) .The volume of the gas mixture is 1.7 L and its total pressure is 0.810 atm. -Determine the number of moles of hydrogen gas present in the sample.
A) 42 mol
B) 0.82 mol
C) 1.3 mol
D) 0.056 mol
E) 22 mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What volume of carbon dioxide measured at STP will be formed by the reaction of 1.47 mol of oxygen with 0.900 mol of ethyl alcohol,CH3CH2OH?
A) 40.3 mL
B) 22.0 L
C) 32.9 L
D) 49.4 L
E) 0.980 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Into a 2.22-liter container at 25°C are placed 1.23 moles of O2 gas and 3.20 moles of solid C (graphite) .If the carbon and oxygen react completely to form CO(g) ,what will be the final pressure in the container at 25°C?
A) 27.1 atm
B) 13.5 atm
C) 2.27 atm
D) 35.2 atm
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The valve between the 2.00-L bulb,in which the gas pressure is 1.80 atm,and the 3.00-L bulb,in which the gas pressure is 3.00 atm,is opened.What is the final pressure in the two bulbs,the temperature remaining constant? 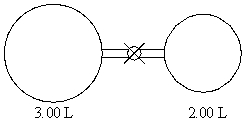
A) 0.720 atm
B) 2.28 atm
C) 2.52 atm
D) 1.80 atm
E) 2.40 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calcium hydride combines with water according to the equation: 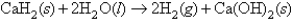 Beginning with 84.0 g of CaH2 and 42.0 g of H2O,what volume of H2 will be produced at 273 K and a pressure of 1327 torr?
Beginning with 84.0 g of CaH2 and 42.0 g of H2O,what volume of H2 will be produced at 273 K and a pressure of 1327 torr?
A) 29.9 L
B) 15.0 L
C) ![]() L
L
D) 25.7 L
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Zinc metal is added to hydrochloric acid to generate hydrogen gas,which is collected over a liquid whose vapor pressure is the same as pure water at 20.0°C (18 torr) .The volume of the gas mixture is 1.7 L and its total pressure is 0.810 atm. -Determine the partial pressure of the hydrogen gas in this mixture.
A) 562 torr
B) 580 torr
C) 598 torr
D) 616 torr
E) 634 torr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You are holding two balloons,an orange balloon and a blue balloon.The orange balloon is filled with neon (Ne) gas and the blue balloon is filled with argon (Ar) gas.The orange balloon has twice the volume of the blue balloon.Which of the following best represents the mass ratio of Ne:Ar in the balloons?
A) 1:1
B) 1:2
C) 2:1
D) 1:3
E) 3:1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would have a higher rate of effusion than C2H2?
A) N2
B) O2
C) Cl2
D) CH4
E) CO2
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 132
Related Exams