A) phospholipids
B) nucleic acids
C) proteins
D) amylose
E) both phospholipids and nucleic acids
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a monomer/polymer pairing?
A) monosaccharide/polysaccharide
B) amino acid/protein
C) triglyceride/phospholipid bilayer
D) deoxyribonucleotide/DNA
E) ribonucleotide/RNA
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The element present in all organic molecules is
A) hydrogen.
B) oxygen.
C) carbon.
D) nitrogen.
E) phosphorus.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
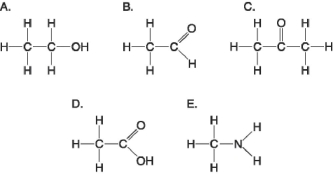 Figure 3.5
-Which molecule shown in Figure 3.5 contains a carboxyl group?
Figure 3.5
-Which molecule shown in Figure 3.5 contains a carboxyl group?
A) A
B) B
C) C
D) D
E) E
Correct Answer

verified
Correct Answer
verified
Multiple Choice
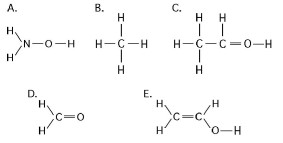 Figure 3.3
-Which of the structures illustrated in Figure 3.3 is an impossible covalently bonded molecule?
Figure 3.3
-Which of the structures illustrated in Figure 3.3 is an impossible covalently bonded molecule?
A) A
B) B
C) C
D) D
E) E
Correct Answer

verified
Correct Answer
verified
Multiple Choice
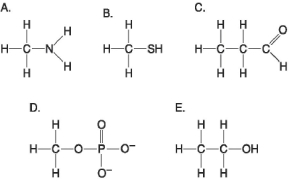 Figure 3.6
-Which molecule shown in Figure 3.6 can form a cross linkage?
Figure 3.6
-Which molecule shown in Figure 3.6 can form a cross linkage?
A) A
B) B
C) C
D) D
E) E
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Lactose, a sugar in milk, is composed of one glucose molecule joined by a glycosidic linkage to one galactose molecule. How is lactose classified?
A) as a pentose
B) as a hexose
C) as a monosaccharide
D) as a disaccharide
E) as a polysaccharide
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What determines whether a carbon atom's covalent bonds to other atoms are in a tetrahedral configuration or a planar configuration?
A) the presence or absence of bonds with oxygen atoms
B) the presence or absence of double bonds between the carbon atom and other atoms
C) the polarity of the covalent bonds between carbon and other atoms
D) the presence or absence of bonds with nitrogen atoms
E) the solvent that the organic molecule is dissolved in
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If cells are grown in a medium containing radioactive 35S, which of these molecules will be labeled?
A) phospholipids
B) nucleic acids
C) proteins
D) amylose
E) both proteins and nucleic acids
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule with the chemical formula C6H12O6 is probably a
A) carbohydrate.
B) lipid.
C) monosaccharide
D) carbohydrate and lipid only.
E) carbohydrate and monosaccharide only.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
 Figure 3.5
-Which molecule shown in Figure 3.5 has a carbonyl functional group in the form of a ketone?
Figure 3.5
-Which molecule shown in Figure 3.5 has a carbonyl functional group in the form of a ketone?
A) A
B) B
C) C
D) D
E) E
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a DNA sample were composed of 10% thymine, what would be the percentage of guanine?
A) 10
B) 20
C) 40
D) 80
E) impossible to tell from the information given
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following categories includes all others in the list?
A) monosaccharide
B) disaccharide
C) starch
D) carbohydrate
E) polysaccharide
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electron pairs does carbon share in order to complete its valence shell?
A) 1
B) 2
C) 3
D) 4
E) 8
Correct Answer

verified
Correct Answer
verified
Multiple Choice
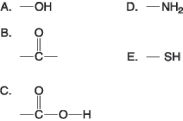 Figure 3.4
-Which of the groups in Figure 3.4 is an acidic functional group that can dissociate and release H+ into a solution?
Figure 3.4
-Which of the groups in Figure 3.4 is an acidic functional group that can dissociate and release H+ into a solution?
A) A
B) B
C) C
D) D
E) E
Correct Answer

verified
Correct Answer
verified
Multiple Choice
 Figure 3.5
-Which molecule shown in Figure 3.5 has a carbonyl functional group in the form of an aldehyde?
Figure 3.5
-Which molecule shown in Figure 3.5 has a carbonyl functional group in the form of an aldehyde?
A) A
B) B
C) C
D) D
E) E
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best summarizes the relationship between dehydration reactions and hydrolysis?
A) Dehydration reactions assemble polymers, and hydrolysis reactions break down polymers.
B) Dehydration reactions eliminate water from lipid membranes, and hydrolysis makes lipid membranes water permeable.
C) Dehydration reactions can occur only after hydrolysis.
D) Hydrolysis creates monomers, and dehydration reactions break down polymers.
E) Dehydration reactions ionize water molecules and add hydroxyl groups to polymers; hydrolysis reactions release hydroxyl groups from polymers.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which level of protein structure do the α helix and the β pleated sheet represent?
A) primary
B) secondary
C) tertiary
D) quaternary
E) primary, secondary, tertiary, and quaternary
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The enzyme amylase can break glycosidic linkages between glucose monomers only if the monomers are the α form. Which of the following could amylase break down?
A) starch
B) cellulose
C) chitin
D) starch and chitin only
E) starch, cellulose, and chitin
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Research indicates that ibuprofen, a drug used to relieve inflammation and pain, is a mixture of two enantiomers, that is, molecules that
A) have identical chemical formulas but differ in the branching of their carbon skeletons.
B) are mirror images of one another.
C) exist in either linear chain or ring forms.
D) differ in the location of their double bonds.
E) differ in the arrangement of atoms around their double bonds.
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 121
Related Exams