A) I only
B) II only
C) III only
D) II and III only
E) Reactants and products can never be in equilibrium in their standard states.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The entropy of an oxygen molecule in air corresponds to approximately 1 1010 accessible states. Which of the following states contribute most to this entropy? I. translational II. rotational III. vibrational IV. electronic
A) I and II
B) I and III
C) I and IV
D) II and III
E) III and IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The prediction of linearity in a van 't Hoff plot assumes ________
A) that the reaction is run under ideal conditions.
B) that H and S are independent of temperature.
C) that G is independent of temperature.
D) that ln K is independent of temperature.
E) none of the above.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The symbol 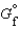 (NH4NO3, s) refers to which of the following reactions?
(NH4NO3, s) refers to which of the following reactions?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following processes is/are spontaneous? I. Iron in the open air rusts. II. Liquid water in a freezer turns to ice. III. A spark ignites a mixture of propane and air.
A) I only
B) I and II only
C) I and III only
D) II and III only
E) I, II, and III are all spontaneous.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The enthalpy and entropy of vaporization of ethanol are 38.6 kJ/mol and 109.8 J/mol · K, respectively. What is the boiling point of ethanol under equilibrium conditions, in C?
A) (352C)
B) (78.5C)
C) (2.84C)
D) (624C)
E) Not enough information is given to answer the question.
Correct Answer

verified
Correct Answer
verified
Showing 181 - 186 of 186
Related Exams