A) A is Cl2, B is AlCl3 and C is meta chloronitrobenzene
B) A is Cl2, B is FeCl3 and C is ortho chloronitrobenzene
C) A is Cl2, B is FeBr3 and C is meta chloronitrobenzene
D) A is HCl, B is AlCl3 and C is para chloronitrobenzene
E) A is Cl2, B is AlBr3 and C is meta chloronitrobenzene
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A hydrocarbon has a molecular weight 56 g/mol and contains 85.7% carbon. It easily adds one mol of HBr to produce a chiral alkyl bromide. Which of the following structures could be our hydrocarbon?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following could be added across carbon-carbon multiple bonds? I. H2SO4 II. H2 III. H2O IV. NO2 V. HBr
A) II, III, and V
B) II, III, and IV
C) I, III, and V
D) I, III, and IV
E) I, II, and III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Hyperconjugation is best described as:
A) an interaction between the orbitals on a substrate and orbitals on a nucleophile
B) an interaction between σ bonds and π network
C) a resonance structure with separated charges
D) an interaction between two π networks
E) an electrostatic interaction between an electrophile and a nucleophile
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Arrange the following alkenes according to their increasing stability: 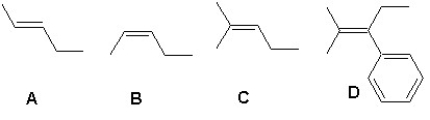
A) A < B < C < D
B) B < C < A < D
C) B < A < C < D
D) C < B < A < D
E) C < A < B < D
Correct Answer

verified
Correct Answer
verified
True/False
Nucleophiles are electron poor atoms, ions or groups
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the major product for the reaction: CH3C≡CH + 2HBr →
A) CH3CBr2CH3
B) CH3CH2CHBr2
C) CH3CHBrCH2Br
D) CH2=CBrCH2Br
E) no reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Oxidation of the following alcohol via the conditions below yields spearmint oil. What new functional group is formed in the reaction to yield spearmint? 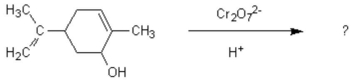
A) aldehyde
B) carboxylic acid
C) ketone
D) amine
E) ester
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Arrange the following carbocations in order of decreasing stability: 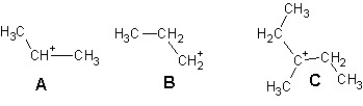
A) A > B > C.
B) C > B > A
C) A > B > C
D) C > A > B.
E) B > A > C.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds could be used as catalysts in halogenation of benzene? I. AlCl3 II. NaCl III. FeBr3 IV. MgCl2 V. PbCl2
A) I) and V)
B) II) and III)
C) II) and IV)
D) I) and III)
E) III) and V)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Markovnikof's rule predicts that when HBr is added to an unsymmetrical alkene or alkyne, the H atom will add to which carbon atom?
A) the carbon with the fewest attached hydrogens
B) the carbon with the Br
C) the carbon with the most attached hydrogens
D) the carbon next to the double or triple bond
E) HBr does not react with unsymmetrical alkenes or alkynes.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When (CH3) 3CBr reacts with CH3ONa in methanol, two products are formed: a major one is an alkene and minor one is an ether. Identify the two products and by what mechanism(s) they are formed?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
The formation of isopropanol (rubbing alcohol) through the following hydrolysis is known as what type of general reaction? 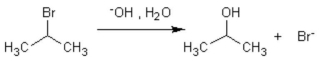
A) oxidation
B) aromatic substitution
C) addition reaction
D) elimination
E) substitution reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Suggest possible compounds for A and B in the following scheme: CH3CH2I + A → CH3CH2SH + B
A) A is HS- and B is I-
B) A is H2S and B is I2
C) A is H2S and B is HI
D) A is S2- and B is I-
E) A is H2S and B is I-
Correct Answer

verified
A
Correct Answer
verified
True/False
Reactions between alkanes and HX compounds are typical examples of addition reactions.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is(are) the main product(s) of the following chlorination reaction? 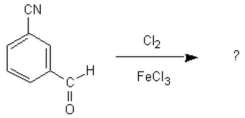
A) 3-chloro-5-cyanobenzaldehyde
B) 2, 6-dichloro-3-cyanobenzaldehyde
C) 2-chloro-3-cyanobenzaldehyde
D) 2-chloro-3-cyanobenzaldehyde and 2-chloro-5-cyanobenzaldehyde
E) B and D
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The initiation step in chain reaction for alkene substitution produces:
A) free halogen radicals
B) free alkyl radicals
C) both free halogen and alkyl radicals
D) free halonium ions
E) free halide ions
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the main product of the following reaction? 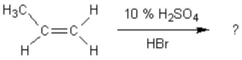
A) 1-bromopropane
B) 2-bromopropane
C) propyl alcohol
D) 2-bromopropene
E) 1-bromo-2-methylethane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the product(s) of the reaction: CH2=CHCH3 + HCl → product(s)
A) CH3CH2CH3 + H2
B) CH2ClCHClCH3 + H2
C) CH3CHClCH3
D) CH2ClCH2CH3
E) none of these
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
What type of polymerization reaction occurred to produce the following polymer? 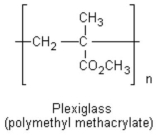
A) step-reaction polymerization
B) elimination reaction
C) chain-reaction polymerization
D) condensation polymerization
E) propagation polymerization
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 94
Related Exams