A) 293°C
B) 20.0°C
C) 160.0°C
D) 2.50°C
E) 10.0°C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Four identical 1.0-L flasks contain the gases He, Cl2, CH4, and NH3, each at 0°C and 1 atm pressure. -For which gas do the molecules have the smallest average kinetic energy?
A) He
B) CH4
C) NH3
D) Cl2
E) The molecules of all the gases have the same average kinetic energy.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 3.82-g sample of lead(II) nitrate, Pb(NO3) 2, molar mass = 331 g/mol, is heated in an evacuated cylinder with a volume of 1.70 L. The salt decomposes when heated, according to the equation 2Pb(NO3) 2(s) → 2PbO(s) + 4NO2(g) + O2(g) Assuming complete decomposition, what is the pressure in the cylinder after decomposition and cooling to a temperature of 290. K? Assume the PbO(s) takes up negligible volume.
A) 0.565 atm
B) 0.162 atm
C) 0.784 atm
D) 0.808 atm
E) 0.404 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mass of 1.12 L of gas Y is found to be 6.23
A) 0.180 g/L.
B) 0.200 g/L.
C) 10.6 g/L.
D) 15.6 g/L.
E) 5.56 g/L.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Air has an average molar mass of 29.0 g/mol. The density of air at 1.00 atm and 30°C is
A) 40.0 g/mL.
B) 1.17 g/L.
C) 1.29 g/L.
D) 12 g/L.
E) 29.0 g/L.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which statement about kinetic energy (K.E.) is true?
A) The K.E. of a body will double if its velocity doubles.
B) As the velocity of a body increases, its K.E. decreases.
C) The K.E. of a body is independent of its mass.
D) All objects moving with the same velocity have the same K.E.
E) None of these statements is true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the following ratios for a gas at Kelvin temperatures T1 and T2 where T2 = 2T1. -Mean free path at T1 : Mean free path at T2
A) 0.50
B) 1.4
C) 2.0
D) 0.71
E) 1.0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Air is 79% N2 and 21% O2 by volume. Calculate the density of air at 1.0 atm, 25°C.
A) 1.18 g/L
B) 2.46 g/L
C) 0.590 g/L
D) 14.1 g/L
E) none of these
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which of the following relationships is not true?
A) PV = constant when temperature and moles of gas are held constant.
B) V/T = constant when pressure and moles of gas are held constant.
C) nT = constant when pressure and volume are held constant.
D) P/n = constant when volume and temperature are held constant.
E) All of these are true.
Correct Answer

verified
Correct Answer
verified
Short Answer
A sample of helium gas has been contaminated with argon gas. At 1 atm and 25°C, the density of the mixture is 0.200 g/L. What is the volume percent argon in the sample?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 275.0-mL sample of O2 is collected over water at 60.0°C. The total pressure is 755 torr. What is the volume of the O2 at the STP? (The vapor pressure of water at 60°C is 149 torr) .
A) 244.0 mL
B) 224.0 mL
C) 180.0 mL
D) 333.0 mL
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which gas, Kr or CO2 , has the lower value of b in the van der Waals equation?
A) The two gases have the same value of
B) It depends on the conditions of pressure and temperature.
C) CO2
D) Kr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The root-mean-square velocity of N2 gas at 35°C is
A) 52.0 m/s.
B) 177 m/s.
C) 5.58 m/s
D) 524 m/s.
E) 16.6 m/s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider three 1-L flasks at the same temperature and pressure. Flask A contains CO gas, flask B contains N2 gas, and flask C contains O2 gas.In which flask do the molecules have the greatest kinetic energy?
A) flask A
B) flask B
C) flask C
D) The molecules in all the flasks have the same kinetic energy.
E) The molecules in two of the flasks have the same kinetic energy.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the following ratios for a gas at Kelvin temperatures T1 and T2 where T2 = 2T1.Average kinetic energy at T1 : Average kinetic energy at T2
A) 2.0
B) 0.50
C) 0.71
D) 1.0
E) 1.4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider two 1.0-L containers, one containing He(g) at 2.50 atm and 24°C and the other containing Ar(g) at 5.00 atm and 48°C. -Calculate the ratio of average velocities (He:Ar) .
A) 0.108
B) 4.98
C) 0.200
D) 9.22
E) none of these
Correct Answer

verified
Correct Answer
verified
Short Answer
A certain element (Z) reacts to form three gaseous compounds. Consider the following data (pressure = 1.0 atm). 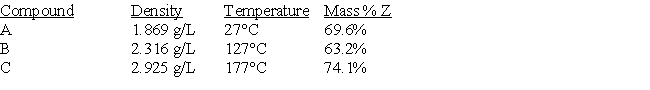 Determine the identity of element Z.
Determine the identity of element Z.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider two samples of helium in separate containers of the same volume. Sample 1 has an absolute temperature four times that of Sample 2. Both samples are at the same pressure.Calculate the ratio n1/n2.
A) 2:1
B) 1:1
C) 1:2
D) 4:1
E) 1:4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A mixture of KCl and KClO3 weighing 1.80 g was heated; the dry O2 generated occupied 1.40 × 102 mL at STP. What percent of the original mixture was KClO3? KClO3 decomposes as follows: 2KClO3(s) → 2KCl(s) + 3O2(g)
A) 37.2%
B) 72.6%
C) 42.6%
D) 63.8%
E) 28.4%
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
Calculate the mean free path in a sample of oxygen gas (M = 3.20 × 10-2 kg/mol) at 35°C and 1.7 atm. Assume that the diameter of an O2 molecule is 300 pm.
A) 0.6 × 10-7 m
B) 0.2 × 10-7 m
C) 1.3 × 10-7 m
D) 2.5 × 10-7 m
E) 3.3 × 10-7 m
Correct Answer

verified
A
Correct Answer
verified
Showing 1 - 20 of 118
Related Exams