A) 1-butanol
B) 2-butanol
C) 2-methyl-1-propanol
D) 2-methyl-2-propanol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the IUPAC name of butyl alcohol?
A) 1-butanol
B) 2-butanol
C) 2-methyl-1-propanol
D) 2-methyl-2-propanol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements is present in thiols but not in alcohols?
A) C
B) O
C) P
D) S
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following alcohols has the lowest boiling point?
A) 1-pentanol
B) 1-octanol
C) 1-hexanol
D) 1-heptanol
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
What is the maximum number of hydrogen bonds which can be formed by a single molecule of an alcohol which contains only one hydroxyl group?
A) 1
B) 2
C) 3
D) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Two commonly used inhalation anesthetics are Ethrane and Forane. They are two of many isomeric ethers which have the molecular formula C3H2ClF5O. In how many of these isomeric ethers are three F atoms bonded to a single carbon atom?
A) 6
B) 8
C) 10
D) 12
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about London dispersion forces is/are true?
A) London dispersion forces are stronger between molecules of 1-butanol than between molecules of 2-methyl-2-propanol.
B) London dispersion forces are weaker than hydrogen bonding interactions.
C) both a and b
D) neither a nor b
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name of the following compound? 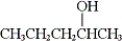
A) methyl propyl methanol
B) 2-pentanol
C) 1-methyl-1-butanol
D) 4-pentanol
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which of the following statements concerning the boiling points of isomeric alcohols is true?
A) Isomeric alcohols have the same boiling points.
B) The relative boiling points of isomeric alcohols are determined primarily by the different number of hydrogen bonds which they can form.
C) The relative boiling points of isomeric alcohols are determined primarily by the different shapes of the molecules.
D) The relative boiling points of isomeric alcohols are determined equally by both b and C )
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of 1-pentanol, 2-pentanol and 3-pentanol, which compound(s) can yield only a single alkene product when dehydrated?
A) only 1-pentanol
B) both 1-pentanol and 2-pentanol
C) both 1-pentanol and 3-pentanol
D) all of them
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Thiols are most closely related to which of the following classes of compounds?
A) alcohols
B) aldehydes
C) carboxylic acids
D) ketones
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name for the following compound? 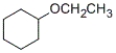
A) cyclohexyl ethyl ether
B) ethyl cyclohexyl ether
C) ethoxycyclohexane
D) cyclohexoxyethane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of the −OCH3 group?
A) methyloxy
B) methoxy
C) oxymethyl
D) oxymeth
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which of the following is the IUPAC name of isobutyl alcohol?
A) 1-butanol
B) 2-butanol
C) 2-methyl-1-propanol
D) 2-methyl-2-propanol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When a primary alcohol is oxidized to a carboxylic acid, an intermediate product is an aldehyde. Which physical property of the aldehyde can be exploited if it is desired to obtain the aldehyde as the reaction product?
A) boiling point
B) molecular weight
C) solubility
D) any of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following explains why the boiling points of ethers are more similar to those of comparable alkanes than to those of comparable alcohols?
A) Both ether molecules and alkane molecules are nonpolar.
B) Hydrogen bonding between ether molecules is weak.
C) Although ether molecules are polar they cannot form hydrogen bonds to other ether molecules.
D) None of the above is the correct explanation.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would be the IUPAC name for the ether commonly called ethyl methyl ether?
A) ethoxymethane
B) ethoxyethane
C) methoxymethane
D) methoxyethane
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is true about the polarities of the C−O and O−H bonds?
A) Neither the C−O nor the O−H bond is polar.
B) The C−O and O−H bonds are equally polar.
C) Both bonds are polar, but the C−O bond is less polar than the O−H bond.
D) Both bonds are polar, but the O−H bond is less polar than the C−O bond.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of oxygen are consumed during the complete combustion of one mole of methanol?
A) 1
B) 1.5
C) 2
D) 2.5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is true of the alcohol functional groups in 2,3-butanediol?
A) One is secondary and one is tertiary.
B) Both are secondary.
C) Both are tertiary.
D) None of the above is true.
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 122
Related Exams