A) 1) OsO4; 2) NaHSO3, H2O
B) 1) Hg(OAc) 2, H2O; 2) NaBH4
C) H2, Pt
D) 1) BH3∙THF; 2) H2O2, NaOH
E) 1) O3; 2) DMS
Correct Answer

verified
Correct Answer
verified
Essay
Propose a multi-step synthetic sequence to accomplish the transformation below. 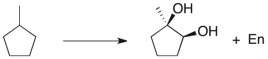
Correct Answer

verified
1) Br2, h...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Devise an efficient synthesis of the compound shown below starting with 4-methyl-2-pentanol. 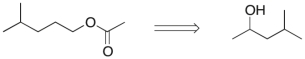
Correct Answer

verified
Correct Answer
verified
Essay
Propose a three-step synthetic sequence to accomplish the transformation below. 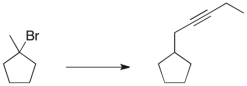
Correct Answer

verified
1) tBuOK 2...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Predict the major product(s) for the reaction of pent-1-en-4-yne with sodium amide followed by reaction with bromoethane.
A) Hept-1-en-4-yne
B) Hept-6-en-3-yne
C) Hept-3-en-6-yne
D) Hept-4-en-1-yne
Correct Answer

verified
Correct Answer
verified
Essay
Propose an efficient synthesis of propanoic acid from acetylene:
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the transformation shown, select the most appropriate reagent(s) to effect the change. 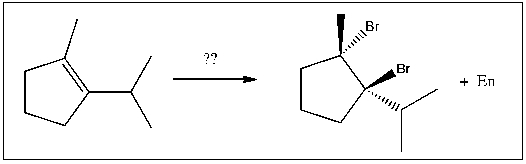
A) HBr
B) Br2/h
C) Br2
D) HBr/ROOR
E) h /NBS
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the minimum number of steps required to convert 2-methylpropane into 1-bromo-2-methyl-2-propanol?
A) 1
B) 2
C) 3
D) 4
E) 5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the transformation shown, select the most appropriate reagent(s) to effect the change. 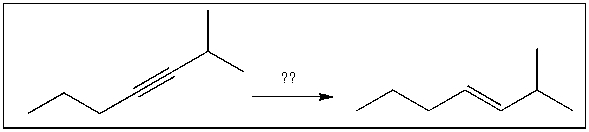
A) 1) OsO4; 2) NaHSO3, H2O
B) NaNH2
C) H2, Pt
D) Na, NH3(l)
E) H2, Lindlar's catalyst
Correct Answer

verified
Correct Answer
verified
Essay
Propose a multi-step synthetic sequence to accomplish the transformation below. 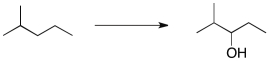
Correct Answer

verified
1) Br2, h...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Show that you understand the concept of retrosynthetic analysis by working backwards two steps in the synthesis below. Identify possible combinations of A and B that can lead to the alkene (C) : 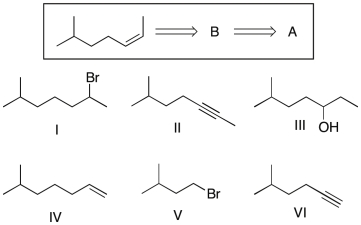
A) B = I and A = IV
B) B = II and A = VI
C) B = III and A = I
D) B = I and A = VI
E) B = III and A = II
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following sequences efficiently converts 2-methylpropene and sodium acetylide into 3-methylbutanal? 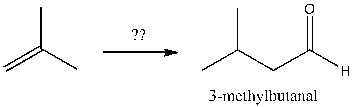
A) 1) HBr; 2) NaCCH; 3) O3; 4) H2O
B) 1) HBr; 2) NaCCH; 3) O3; 4) DMS
C) 1) HBr, ROOR; 2) NaCCH; 3) O3; 4) H2O
D) 1) HBr, ROOR; 2) NaCCH; 3) H2/Ni2B 4) O3; 5) DMS
E) 1) NaCCH; 2) H2/Ni2B; 3) O3; 4) DMS
Correct Answer

verified
Correct Answer
verified
Essay
Devise a method to complete the following synthesis. 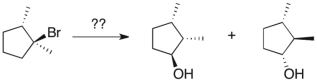
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the transformation shown, select the most appropriate reagent(s) to effect the change. 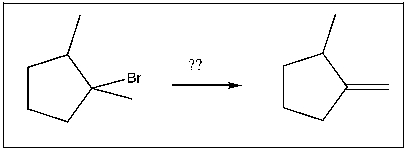
A) NaOtBu
B) HBr
C) H2SO4
D) NaOH
E) NaSH
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Provide the major product(s) obtained from the following reaction: 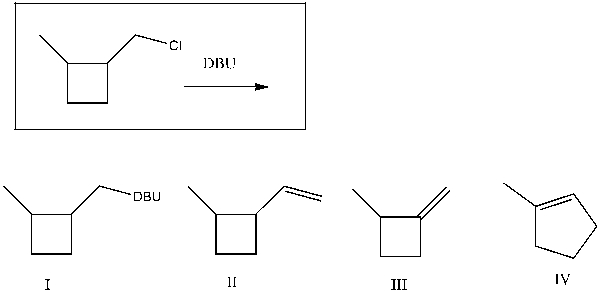
A) I
B) II
C) III
D) IV
E) II and IV
Correct Answer

verified
Correct Answer
verified
Essay
One compound is produced when acetylene is treated with the following reagents. What is the product? 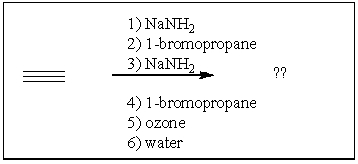
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the major product(s) for the following reaction: 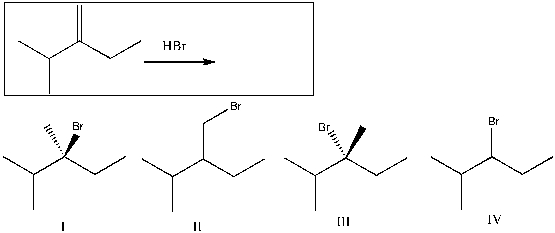
A) I
B) II
C) III
D) IV
E) I and III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Show that you understand the concept of retrosynthetic analysis by working backwards two steps in the synthesis below. Identify possible combinations of A and B that can lead to the compound shown (C) : 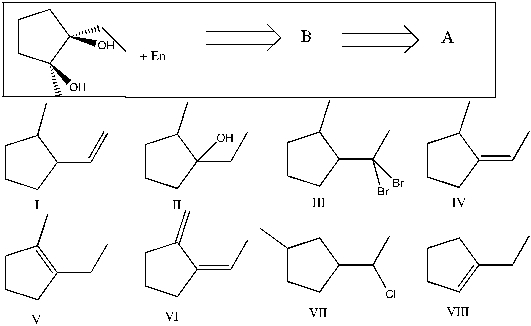
A) B = I and A = VI
B) B = V and A = II
C) B = IV and A = VII
D) B = I and A = III
E) B = VIII and A = V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following sequences of reagents will move the alcohol functional group from the tertiary position of 1-methyl-1-cyclohexanol to a secondary position?
A) 1) KOtBu; 2) Hg(OAc) 2, H2O; 3) NaBH4
B) 1) TsCl, pyr; 2) KOtBu; 3) BH3-THF; 4) H2O2, NaOH
C) 1) H2SO4, heat; 2) BH3∙THF; 3) H2O2, NaOH
D) 1) TsCl, pyr; 2) NaOH; 3) BH3∙THF; 4) H2O2, NaOH
E) C and D will both work
Correct Answer

verified
Correct Answer
verified
Essay
Devise a method to complete the following synthesis. 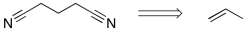
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 95
Related Exams