A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is expected to be a major product for the reaction shown below? 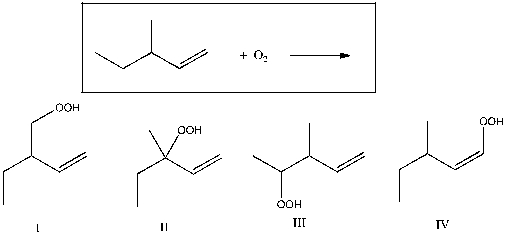
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Essay
Propose an efficient synthesis of 2-methylpropene from 2-methylpropane.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the product(s) of the following reaction: 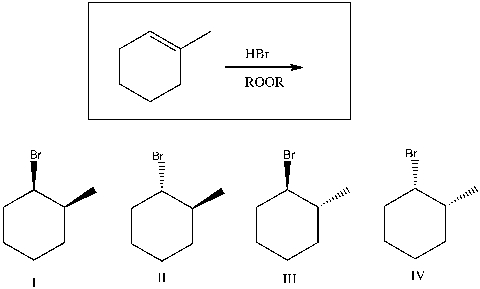
A) I
B) II
C) III
D) I, II
E) I, II, III, IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which term best describes the process shown below? 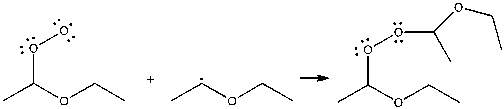
A) initiation
B) propagation
C) termination
D) elimination
E) inhibition
Correct Answer

verified
Correct Answer
verified
Essay
Azobisisobutyronitrile (AIBN) is commonly used as a radical initiator. Use correct arrow formalism to show this process. 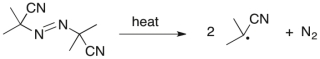
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the major product obtained upon radical bromination of t-butylcyclohexane.
A) 1-bromo-1-tert-butylcyclohexane
B) 2-bromo-1-tert-butylcyclohexane
C) 3-bromo-1-tert-butylcyclohexane
D) 4-bromo-1-tert-butylcyclohexane
E) (1-bromo-1,1-dimethyl) ethylcyclohexane
Correct Answer

verified
Correct Answer
verified
Essay
Draw the major product(s) of the following reaction. Is the product optically active? Explain. 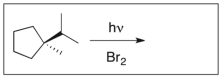
Correct Answer

verified
 The product is not ...
The product is not ...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Draw the product of coupling of the following radicals. 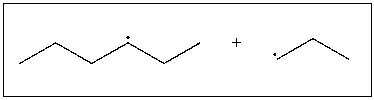
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the major product(s) of the following reaction:
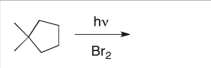
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Essay
Predict the major product(s) of the following reaction. 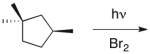
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would you expect to function as an initiator at the lowest temperature?
A) ![]()
B) Cl2
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the major product(s) of the following reaction. 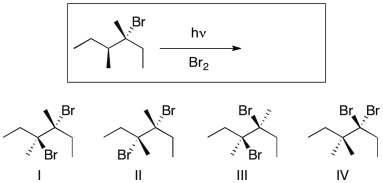
A) I
B) I and II
C) I, II, and III
D) I, II, III
E) IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following correctly describes the nature of the transition state of the rate-determining step of the free-radical bromination of methane?
A) the transition state resembles the reactants more than the products
B) the transition state resembles the products more than the reactants
C) the transition state equally resembles products and reactants
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following shows the correct products initially formed (first step) when ozone absorbs ultraviolet light?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which term most accurately describes the process shown below? 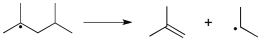
A) coupling
B) elimination
C) halogen abstraction
D) hydrogen abstraction
E) homolytic cleavage
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the labeled C-H bonds is the weakest? 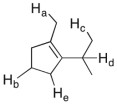
A) C-Ha
B) C-Hb
C) C-Hc
D) C-Hd
E) C-He
Correct Answer

verified
Correct Answer
verified
Essay
Propose an efficient synthesis of 1-iodo-2-methylpropane from 2-methylpropene.
Correct Answer

verified
Correct Answer
verified
Essay
Use correct arrow formalism to show the mechanism of the following radical process: 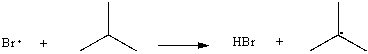
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following steps is thermodynamically unfavorable at all temperatures?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 90
Related Exams