Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular geometry of NO2- as predicted by the VSEPR model?
A) linear
B) trigonal planar
C) bent
D) tetrahedral
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the total number of electron domains around the central atom for a molecule having a square planar molecular geometry,such as XeBr4?
A) 2
B) 3
C) 4
D) 5
E) 6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is true concerning the molecular geometry of SF6?
A) The predicted F-S-F bond angle is 60°.
B) The predicted F-S-F bond angle is 90°.
C) The predicted F-S-F bond angles are 90° and 120°.
D) The predicted F-S-F bond angles are 90° and 180°.
E) The predicted F-S-F bond angles are 60°,120°,and 180°.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A molecule with the formula AB4 and a tetrahedral molecular geometry uses _________ to form its σ bonds.
A) sp hybrid orbitals
B) sp2 hybrid orbitals
C) sp3 hybrid orbitals
D) sp3d hybrid orbitals
E) sp3d2 hybrid orbitals
Correct Answer

verified
Correct Answer
verified
Short Answer
The number of lone pairs on the central atom is used to determine the ____________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the most reasonable prediction for the H-O-H bond angle in H2O?
A) 90°
B) 109.5°
C) 120°
D) 107°
E) 105°
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following molecules has a zero dipole moment?
A) CO
B) CH2Cl2
C) SO3
D) SO2
E) NH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the total number of electron domains around the central atom for a molecule having a square pyramidal molecular geometry,such as ClF5?
A) 5
B) 2
C) 3
D) 4
E) 6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the number of lone electron pairs on the central atom of a molecule having a linear molecular geometry,such as CO2?
A) 1
B) 2
C) 3
D) 0
E) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the VSEPR model,what is the predicted electron-domain geometry around the central iodine atom in the ion lF2- ?
A) octahedral
B) trigonal bipyramidal
C) tetrahedral
D) trigonal planar
E) bent
Correct Answer

verified
Correct Answer
verified
True/False
A molecule which contains polar bonds will always have a dipole moment.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to molecular orbital (MO) theory,the twelve valence electrons in the O2 molecule are distributed as follows:
A) 12 in bonding MOs,0 in antibonding MOs.
B) 10 in bonding MOs,2 in antibonding MOs.
C) 9 in bonding MOs,3 in antibonding MOs.
D) 8 in bonding MOs,4 in antibonding MOs.
E) 7 in bonding MOs,5 in antibonding MOs.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the total number of electron domains around the central atom for a molecule having a trigonal pyramidal molecular geometry,such as NH3?
A) 5
B) 2
C) 3
D) 4
E) 6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the species N2-,N2,and N2+.Which of these species will be paramagnetic?
A) N2 and N2-
B) N2+ and N2
C) N2+ and N2-
D) N2-,N2,and N2+
E) None is paramagnetic.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the VSEPR model,a molecule with the general formula AB3 with no lone pairs on the central atom will have a ______ molecular shape.
A) linear
B) bent
C) trigonal planar
D) tetrahedral
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The PCl5 molecule has
A) nonpolar bonds,and is a nonpolar molecule.
B) nonpolar bonds,but is a polar molecule.
C) polar bonds,and is a polar molecule.
D) polar bonds,but is a nonpolar molecule.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Indicate the type of hybrid orbitals used by the central atom in SF6.
A) sp
B) sp2
C) sp3
D) sp3d
E) sp3d2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many π bonds are there in one molecule of acrylonitrile (shown below) ? 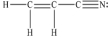
A) 1
B) 2
C) 3
D) 5
E) 9
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecular formula corresponds to a structural formula with carbon atoms having hybridizations of sp,sp2,and sp3?
A) C3H6
B) C4H4
C) C4H6
D) C5H6
E) C5H8
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 137
Related Exams