Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction CH3NC(g) → CH3CN(g) is first order with respect to methyl isocyanide,CH3NC.If it takes 10.3 minutes for exactly one quarter of the initial amount of methyl isocyanide to react,what is the rate constant in units of min-1?
A) -0.135 min-1
B) 0.0279 min-1
C) 0.089 min-1
D) 0.135 min-1
E) 35.8 min-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rate constant for the reaction 3A → 4B is 6.00 × 10-3 L • mol-1 • min-1.How long will it take the concentration of A to drop from 0.75 M to 0.25 M?
A) 2.2 × 10-3 min
B) 5.5 × 10-3 min
C) 180 min
D) 440 min
E) 5.0 × 102min
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the half-life for a first-order reaction?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Short Answer
A(n)_______________ is the name given to a collision that does result in a reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider reactions A,B,and C,which have the potential energy profiles shown.Assuming that the reactions have roughly the same frequency factors,which reaction is the slowest? 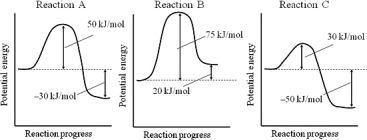
A) Reaction A
B) Reaction B
C) Reaction C
D) All of the reactions have the same rate.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction A + 2B → products has been found to have the rate law,rate = k[A][B]2.While holding the concentration of A constant,the concentration of B is increased to three times its initial value.By what factor does the rate of reaction increase?
A) 3
B) 6
C) 9
D) 27
E) 30
Correct Answer

verified
Correct Answer
verified
Multiple Choice
It takes 42.0 min for the concentration of a reactant in a first-order reaction to drop from 0.45 M to 0.32 M at 25°C.How long will it take for the reaction to be 90% complete?
A) 13.0 min
B) 86.0 min
C) 137 min
D) 222 min
E) 284 min
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following diagram represents the zeroth-order decomposition of A to form X according to the following balanced chemical equation: A → X.Each sphere represents 1.0 mmol of atoms,and the volume of the box is 1.0 L. 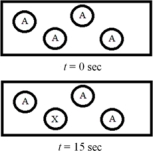 What is the half-life of the reaction?
What is the half-life of the reaction?
A) 15 s
B) 30 s
C) 36 s
D) 45 s
E) 60 s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At 25°C the rate constant for the first-order decomposition of a pesticide solution is 6.40 × 10-3 min-1.If the starting concentration of pesticide is 0.0314 M,what concentration will remain after 62.0 min at 25°C?
A) 1.14 × 10-1 M
B) 47.4 M
C) 1.25 ×10-2 M
D) 2.11 × 10-2 M
E) 2.68 × 10-2 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the half-life for a zeroth-order reaction?
A) t1/2 = k
B) t1/2 = 1/k[A]o
C) t1/2 = 0.693/k[A]o
D) t1/2 = [A]o/2k
E) t1/2 = 0.693/k
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the hypothetical reaction A + 3B → 2C,the rate should be expressed as
A) rate = Δ[A]/Δt.
B) rate = -Δ[C]/Δt.
C) rate = -3(Δ[B]/Δt) .
D) rate = (1/2) (Δ[C]/Δt) .
E) rate = (1/3) (Δ[B]/Δt) .
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the rate expression for the production of X,which is a gas,at constant volume?
A) rate = PgVΔ[X]/RΔt
B) rate = -TΔ[X]/PgVΔt
C) rate = -PgV(Δ[X]/TΔt)
D) rate = (1/RT) (ΔPX/Δt)
E) rate = (1/RΔPX) (VT/Δt)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Cyclopropane is converted to propene in a first-order process.The rate constant is 5.4 × 10-2 h-1.If the initial concentration of cyclopropane is 0.150 M,what will its concentration be after 22.0 hours?
A) 0.046 M
B) 0.11 M
C) 0.13 M
D) 0.49 M
E) 0.054 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sucrose decomposes to fructose and glucose in acid solution.When ln [sucrose] is plotted vs.time,a straight line with slope of -0.208 h-1 results.What is the rate law for the reaction?
A) Rate = (0.208 h-1) [sucrose]2
B) Rate = (0.208 h-1) [sucrose]
C) Rate = (0.0433 h) [sucrose]2
D) Rate = (0.0433 h) [sucrose]
E) Rate = (0.208 mol L-1 • h-1) [sucrose]0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name given to the slowest step in the mechanism of a reaction?
A) Catalytic step
B) Mechanistic step
C) Rate-determining step
D) Step 1
E) Rate step
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rate law for the rearrangement of CH3NC to CH3CN at 800 K is rate = (1300 s-1) [CH3NC]. What is the half-life for this reaction?
A) 7.69 × 10-4 s
B) 5.3 × 10-4 s
C) 1.9 × 10-3 s
D) 520 s
E) 1920 s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nitric oxide gas (NO) reacts with chlorine gas according to the equation ![Nitric oxide gas (NO) reacts with chlorine gas according to the equation The following initial rates of reaction have been measured for the given reagent concentrations. Cl<sub>2</sub> (M) A) rate = k[NO] B) rate = k[NO][Cl<sub>2</sub>]<sup>1/2</sup> C) rate = k[NO][Cl<sub>2</sub>] D) rate = k[NO]<sup>2</sup>[Cl<sub>2</sub>] E) rate = k[NO]<sup>2</sup>[Cl<sub>2</sub>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB6485/11eab46b_9d23_54d2_a889_2f09b76029a1_TB6485_11.jpg) The following initial rates of reaction have been measured for the given reagent concentrations. Cl2 (M)
The following initial rates of reaction have been measured for the given reagent concentrations. Cl2 (M) ![Nitric oxide gas (NO) reacts with chlorine gas according to the equation The following initial rates of reaction have been measured for the given reagent concentrations. Cl<sub>2</sub> (M) A) rate = k[NO] B) rate = k[NO][Cl<sub>2</sub>]<sup>1/2</sup> C) rate = k[NO][Cl<sub>2</sub>] D) rate = k[NO]<sup>2</sup>[Cl<sub>2</sub>] E) rate = k[NO]<sup>2</sup>[Cl<sub>2</sub>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB6485/11eab46b_9d23_54d3_a889_4f6218f35b92_TB6485_11.jpg)
![Nitric oxide gas (NO) reacts with chlorine gas according to the equation The following initial rates of reaction have been measured for the given reagent concentrations. Cl<sub>2</sub> (M) A) rate = k[NO] B) rate = k[NO][Cl<sub>2</sub>]<sup>1/2</sup> C) rate = k[NO][Cl<sub>2</sub>] D) rate = k[NO]<sup>2</sup>[Cl<sub>2</sub>] E) rate = k[NO]<sup>2</sup>[Cl<sub>2</sub>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB6485/11eab46b_9d23_7be4_a889_b3a5524d1e8d_TB6485_11.jpg)
![Nitric oxide gas (NO) reacts with chlorine gas according to the equation The following initial rates of reaction have been measured for the given reagent concentrations. Cl<sub>2</sub> (M) A) rate = k[NO] B) rate = k[NO][Cl<sub>2</sub>]<sup>1/2</sup> C) rate = k[NO][Cl<sub>2</sub>] D) rate = k[NO]<sup>2</sup>[Cl<sub>2</sub>] E) rate = k[NO]<sup>2</sup>[Cl<sub>2</sub>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB6485/11eab46b_9d23_a2f5_a889_dfc5a0a2432f_TB6485_11.jpg)
![Nitric oxide gas (NO) reacts with chlorine gas according to the equation The following initial rates of reaction have been measured for the given reagent concentrations. Cl<sub>2</sub> (M) A) rate = k[NO] B) rate = k[NO][Cl<sub>2</sub>]<sup>1/2</sup> C) rate = k[NO][Cl<sub>2</sub>] D) rate = k[NO]<sup>2</sup>[Cl<sub>2</sub>] E) rate = k[NO]<sup>2</sup>[Cl<sub>2</sub>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB6485/11eab46b_9d23_a2f6_a889_55f701637f55_TB6485_11.jpg)
A) rate = k[NO]
B) rate = k[NO][Cl2]1/2
C) rate = k[NO][Cl2]
D) rate = k[NO]2[Cl2]
E) rate = k[NO]2[Cl2]2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following is an Arrhenius plot of a first-order reaction.The rate constant is measured in units of s-1. 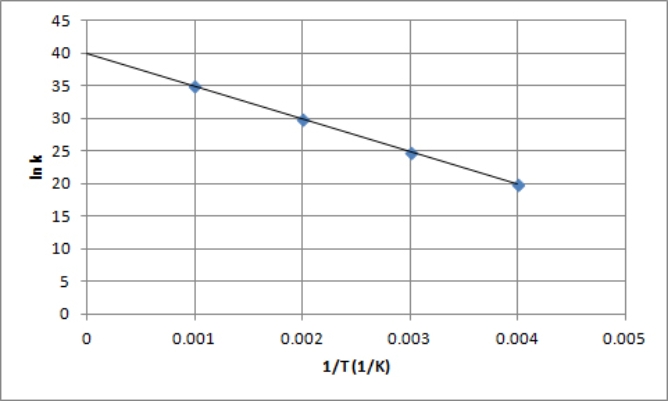 Based on this Arrhenius plot,what is the Arrhenius frequency factor (A) of the reaction? (R =8.314 J/K mol)
Based on this Arrhenius plot,what is the Arrhenius frequency factor (A) of the reaction? (R =8.314 J/K mol)
A) 5.0 s-1
B) 40 s-1
C) 50 s-1
D) 5.0 × 103 s-1
E) 2.4 × 1017 s-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the correct unit for a second-order rate constant?
A) s-1
B) M • s-1
C) M • s
D) M-1 • s-1
E) M-2 • s-1
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 114
Related Exams